

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM26350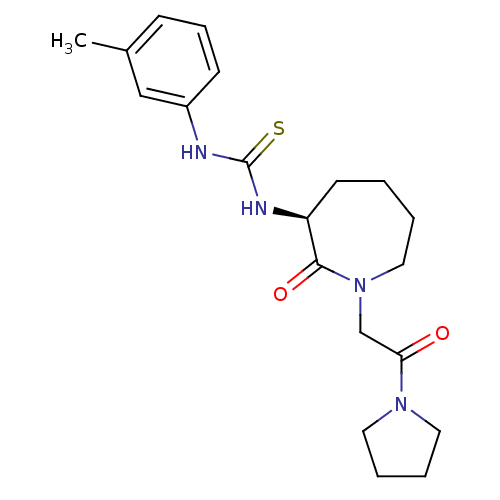 ((S)-1-(2-oxo-1-(2-oxo-2-(pyrrolidin-1-yl)ethyl)aze...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description Time-dependent optical density change was followed at 405 nm using a kinetic microplate reader at room temperature. Enzyme activity in the presence o... | J Med Chem 51: 7541-51 (2008) Article DOI: 10.1021/jm800855x BindingDB Entry DOI: 10.7270/Q2V12332 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM26350 ((S)-1-(2-oxo-1-(2-oxo-2-(pyrrolidin-1-yl)ethyl)aze...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description Time-dependent optical density change was followed at 405 nm using a kinetic microplate reader at room temperature. Enzyme activity in the presence o... | Bioorg Med Chem Lett 19: 6882-9 (2009) Article DOI: 10.1016/j.bmcl.2009.10.084 BindingDB Entry DOI: 10.7270/Q2Z899R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM26350 ((S)-1-(2-oxo-1-(2-oxo-2-(pyrrolidin-1-yl)ethyl)aze...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of factor 10a | J Med Chem 54: 2529-91 (2011) Article DOI: 10.1021/jm1013693 BindingDB Entry DOI: 10.7270/Q24M95PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM26350 ((S)-1-(2-oxo-1-(2-oxo-2-(pyrrolidin-1-yl)ethyl)aze...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 10a-mediated cleavage of synthetic substrate S-2222 | Bioorg Med Chem Lett 19: 4034-41 (2009) Article DOI: 10.1016/j.bmcl.2009.06.014 BindingDB Entry DOI: 10.7270/Q2TQ61KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM26350 ((S)-1-(2-oxo-1-(2-oxo-2-(pyrrolidin-1-yl)ethyl)aze...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human FXa | Bioorg Med Chem Lett 15: 5453-8 (2005) Article DOI: 10.1016/j.bmcl.2005.08.107 BindingDB Entry DOI: 10.7270/Q2KH0MW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||