

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM7552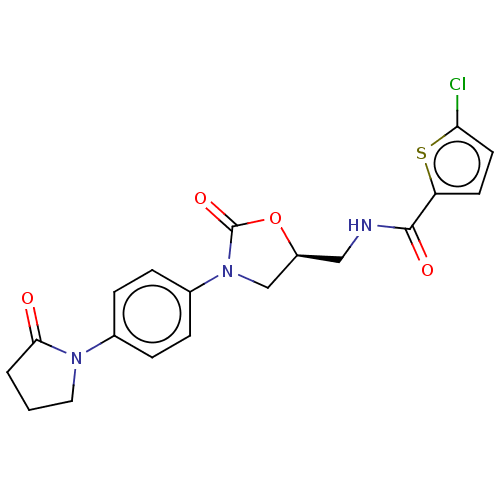 (BAY 59-7939 Analog 11 | US8822458, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | 25 |
Bayer Intellectual Property GmbH US Patent | Assay Description The enzymatic activity of human factor Xa (FXa) was measured using the conversion of a chromogenic substrate specific for FXa. Factor Xa cleaves p-ni... | US Patent US8822458 (2014) BindingDB Entry DOI: 10.7270/Q2VH5MJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM7552 (BAY 59-7939 Analog 11 | US8822458, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of factor 10a | J Med Chem 53: 6243-74 (2010) Article DOI: 10.1021/jm100146h BindingDB Entry DOI: 10.7270/Q2CR5VBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM7552 (BAY 59-7939 Analog 11 | US8822458, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 8.3 | 25 |
Bayer HealthCare AG | Assay Description The enzymatic activity was measured using chromogenic or fluorogenic substrates in 96-well microtiter plates.Color change was monitored continuously ... | J Med Chem 48: 5900-8 (2005) Article DOI: 10.1021/jm050101d BindingDB Entry DOI: 10.7270/Q2J38QR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||