

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyclin-T1 (Homo sapiens (Human)) | BDBM126488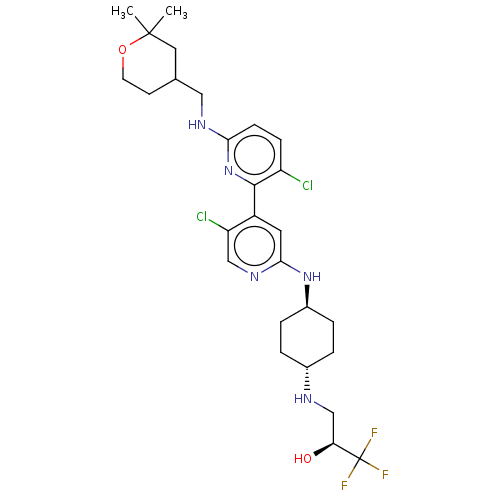 (US8778951, 298) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Cdk9/cyclinT1 is purchased from Millipore, cat #14-685. The final total protein concentration in the assay 4 nM. The 5TAMRA-cdk7tide peptide substrat... | US Patent US8778951 (2014) BindingDB Entry DOI: 10.7270/Q25B014T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||