 Found 6 hits Enz. Inhib. hit(s) with Target = 'Cyclin-dependent kinase 9' and Ligand = 'BDBM164883'
Found 6 hits Enz. Inhib. hit(s) with Target = 'Cyclin-dependent kinase 9' and Ligand = 'BDBM164883' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM164883
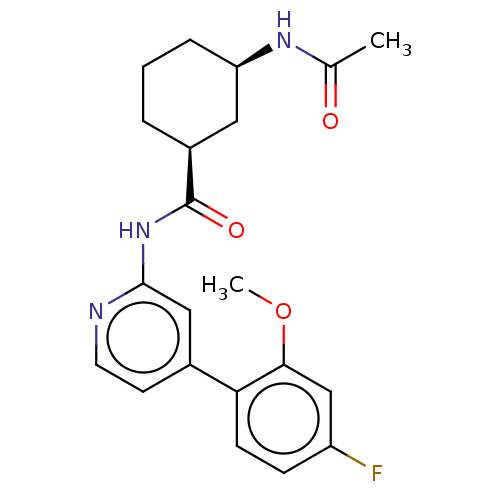
(US9067888, 33)Show SMILES COc1cc(F)ccc1-c1ccnc(NC(=O)[C@H]2CCC[C@H](C2)NC(C)=O)c1 |r| Show InChI InChI=1S/C21H24FN3O3/c1-13(26)24-17-5-3-4-15(10-17)21(27)25-20-11-14(8-9-23-20)18-7-6-16(22)12-19(18)28-2/h6-9,11-12,15,17H,3-5,10H2,1-2H3,(H,24,26)(H,23,25,27)/t15-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
In vitro kinase assay analysis may be performed using standard techniques described in the art. These techniques are also used by commercial services... |
US Patent US9067888 (2015)
BindingDB Entry DOI: 10.7270/Q2CR5S4T |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM164883

(US9067888, 33)Show SMILES COc1cc(F)ccc1-c1ccnc(NC(=O)[C@H]2CCC[C@H](C2)NC(C)=O)c1 |r| Show InChI InChI=1S/C21H24FN3O3/c1-13(26)24-17-5-3-4-15(10-17)21(27)25-20-11-14(8-9-23-20)18-7-6-16(22)12-19(18)28-2/h6-9,11-12,15,17H,3-5,10H2,1-2H3,(H,24,26)(H,23,25,27)/t15-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM164883

(US9067888, 33)Show SMILES COc1cc(F)ccc1-c1ccnc(NC(=O)[C@H]2CCC[C@H](C2)NC(C)=O)c1 |r| Show InChI InChI=1S/C21H24FN3O3/c1-13(26)24-17-5-3-4-15(10-17)21(27)25-20-11-14(8-9-23-20)18-7-6-16(22)12-19(18)28-2/h6-9,11-12,15,17H,3-5,10H2,1-2H3,(H,24,26)(H,23,25,27)/t15-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
ETA receptor antagonist activity was measured by inhibition of ET-1 induced vasoconstriction in isolated porcine coronary artery |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM164883

(US9067888, 33)Show SMILES COc1cc(F)ccc1-c1ccnc(NC(=O)[C@H]2CCC[C@H](C2)NC(C)=O)c1 |r| Show InChI InChI=1S/C21H24FN3O3/c1-13(26)24-17-5-3-4-15(10-17)21(27)25-20-11-14(8-9-23-20)18-7-6-16(22)12-19(18)28-2/h6-9,11-12,15,17H,3-5,10H2,1-2H3,(H,24,26)(H,23,25,27)/t15-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Effective concentration required for inhibitory activity towards human beta-1 adrenergic receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM164883

(US9067888, 33)Show SMILES COc1cc(F)ccc1-c1ccnc(NC(=O)[C@H]2CCC[C@H](C2)NC(C)=O)c1 |r| Show InChI InChI=1S/C21H24FN3O3/c1-13(26)24-17-5-3-4-15(10-17)21(27)25-20-11-14(8-9-23-20)18-7-6-16(22)12-19(18)28-2/h6-9,11-12,15,17H,3-5,10H2,1-2H3,(H,24,26)(H,23,25,27)/t15-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK9 in human MCF7 cells assessed as reduction in RNA Polymerase 2 CTD phosphorylation at Ser2 residues measured after 6 hrs by immunof... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01754
BindingDB Entry DOI: 10.7270/Q2M90D8V |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM164883

(US9067888, 33)Show SMILES COc1cc(F)ccc1-c1ccnc(NC(=O)[C@H]2CCC[C@H](C2)NC(C)=O)c1 |r| Show InChI InChI=1S/C21H24FN3O3/c1-13(26)24-17-5-3-4-15(10-17)21(27)25-20-11-14(8-9-23-20)18-7-6-16(22)12-19(18)28-2/h6-9,11-12,15,17H,3-5,10H2,1-2H3,(H,24,26)(H,23,25,27)/t15-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK9 in human MV4-11 cells assessed as induction of caspase-3/7 activation measured after 6 hrs followed by Caspase-glo reagent based a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01754
BindingDB Entry DOI: 10.7270/Q2M90D8V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data