 Found 6 hits Enz. Inhib. hit(s) with Target = 'Cytochrome P450 2C9' and Ligand = 'BDBM50158460'
Found 6 hits Enz. Inhib. hit(s) with Target = 'Cytochrome P450 2C9' and Ligand = 'BDBM50158460' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50158460
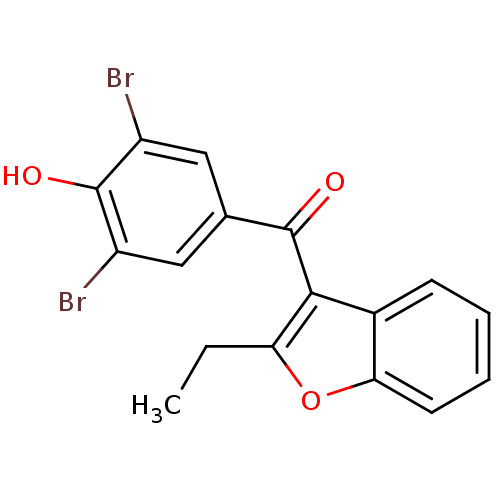
((3,5-dibromo-4-hydroxyphenyl)(2-ethyl-1-benzofuran...)Show InChI InChI=1S/C17H12Br2O3/c1-2-13-15(10-5-3-4-6-14(10)22-13)16(20)9-7-11(18)17(21)12(19)8-9/h3-8,21H,2H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
J Med Chem 47: 6768-76 (2004)
Article DOI: 10.1021/jm049605m
BindingDB Entry DOI: 10.7270/Q29P3153 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50158460

((3,5-dibromo-4-hydroxyphenyl)(2-ethyl-1-benzofuran...)Show InChI InChI=1S/C17H12Br2O3/c1-2-13-15(10-5-3-4-6-14(10)22-13)16(20)9-7-11(18)17(21)12(19)8-9/h3-8,21H,2H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 51: 648-54 (2008)
Article DOI: 10.1021/jm701130z
BindingDB Entry DOI: 10.7270/Q2M32VH2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50158460

((3,5-dibromo-4-hydroxyphenyl)(2-ethyl-1-benzofuran...)Show InChI InChI=1S/C17H12Br2O3/c1-2-13-15(10-5-3-4-6-14(10)22-13)16(20)9-7-11(18)17(21)12(19)8-9/h3-8,21H,2H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Binding affinity to CYP2C9 (unknown origin) |
Bioorg Med Chem 16: 4064-74 (2008)
Article DOI: 10.1016/j.bmc.2008.01.021
BindingDB Entry DOI: 10.7270/Q22N522D |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50158460

((3,5-dibromo-4-hydroxyphenyl)(2-ethyl-1-benzofuran...)Show InChI InChI=1S/C17H12Br2O3/c1-2-13-15(10-5-3-4-6-14(10)22-13)16(20)9-7-11(18)17(21)12(19)8-9/h3-8,21H,2H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127900
BindingDB Entry DOI: 10.7270/Q2P55S6N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50158460

((3,5-dibromo-4-hydroxyphenyl)(2-ethyl-1-benzofuran...)Show InChI InChI=1S/C17H12Br2O3/c1-2-13-15(10-5-3-4-6-14(10)22-13)16(20)9-7-11(18)17(21)12(19)8-9/h3-8,21H,2H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00176
BindingDB Entry DOI: 10.7270/Q2TQ6587 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50158460

((3,5-dibromo-4-hydroxyphenyl)(2-ethyl-1-benzofuran...)Show InChI InChI=1S/C17H12Br2O3/c1-2-13-15(10-5-3-4-6-14(10)22-13)16(20)9-7-11(18)17(21)12(19)8-9/h3-8,21H,2H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tongji University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using tolbutamide substrate by LC-MS/MS method |
Drug Metab Dispos 41: 60-71 (2012)
Article DOI: 10.1124/dmd.112.048264
BindingDB Entry DOI: 10.7270/Q2K0761G |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data