

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM320017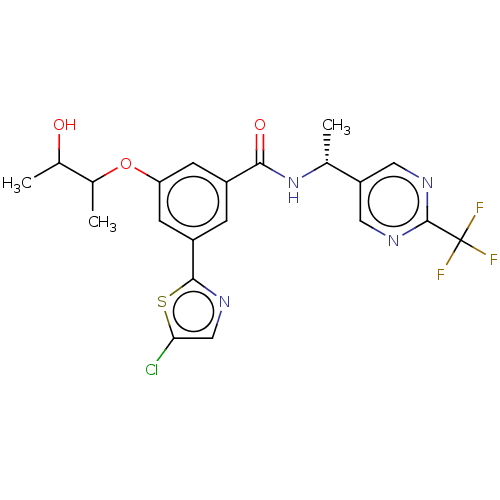 (Trans Isomer 1; 3- (5-chloro-1,3- thiazol-2-yl)-5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Aktiengesellschaft US Patent | Assay Description Human liver microsomes (pooled, >30 male and female donors) were incubated with individual CYP isoform-selective standard probes (phenacetin for CYP1... | US Patent US10202369 (2019) BindingDB Entry DOI: 10.7270/Q2TT4T2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM320017 (Trans Isomer 1; 3- (5-chloro-1,3- thiazol-2-yl)-5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
BEYER AKTIENGESELLSCHAFT US Patent | Assay Description Use of in vitro assays to evaluate the inhibition potential of new drug candidates towards CYP-mediated metabolism has been shown to be effective as ... | US Patent US10174016 (2019) BindingDB Entry DOI: 10.7270/Q2GB264F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||