

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50395571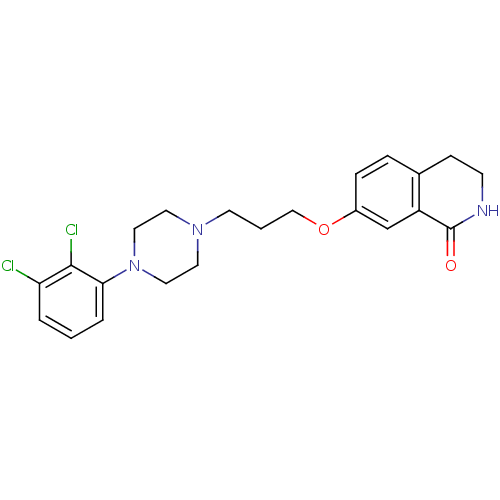 (CHEMBL2165117 | UNC10099981 | US10501452, Compound...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 18 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Membranes prepared as above were resuspended to 1 ug protein/ul (measured by Bradford assay using BSA as standard), and 50 ul were added to each well... | US Patent US9156822 (2015) BindingDB Entry DOI: 10.7270/Q2H41Q6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50395571 (CHEMBL2165117 | UNC10099981 | US10501452, Compound...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human D2L receptor expressed in CHO cells after 1.5 hrs by microbeta counting method | J Med Chem 55: 7141-53 (2012) Article DOI: 10.1021/jm300603y BindingDB Entry DOI: 10.7270/Q2JD4XXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50395571 (CHEMBL2165117 | UNC10099981 | US10501452, Compound...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 21.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NHWA PHARMA. CORPORATION US Patent | Assay Description D2:Procedures(1) The prepared membrane was applied with appropriate amount of buffer, and homogenizer was used for evenly dispersing. 15 tubes were m... | US Patent US10501452 (2019) BindingDB Entry DOI: 10.7270/Q2BK1FR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50395571 (CHEMBL2165117 | UNC10099981 | US10501452, Compound...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Agonist activity at D2L receptor in human HTLA cells assessed as beta arrestin recruitment at 6 uM after 18 hrs by luminescence assay | J Med Chem 55: 7141-53 (2012) Article DOI: 10.1021/jm300603y BindingDB Entry DOI: 10.7270/Q2JD4XXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50395571 (CHEMBL2165117 | UNC10099981 | US10501452, Compound...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 30 | n/a | n/a | 7.4 | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Recruitment of β-arrestin to agonist-stimulated D2 receptors was performed using a previously described Tango-type assay (Barnea et al., Proc. N... | US Patent US9156822 (2015) BindingDB Entry DOI: 10.7270/Q2H41Q6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||