

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50129425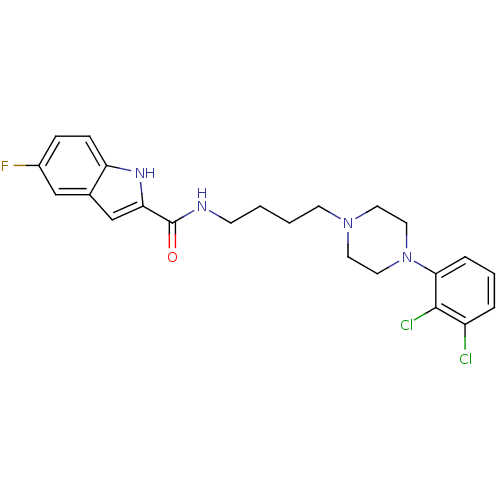 (5-Fluoro-1H-indole-2-carboxylic acid {4-[4-(2,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse- Intramural Research Program Curated by ChEMBL | Assay Description Displacement of [3H]-N-methylspiperone from human dopamine D3 receptor (unknown origin) expressed in HEK293 cells after 1 hr by liquid scintillation ... | J Med Chem 58: 6195-213 (2015) Article DOI: 10.1021/acs.jmedchem.5b00776 BindingDB Entry DOI: 10.7270/Q2KS6T92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50129425 (5-Fluoro-1H-indole-2-carboxylic acid {4-[4-(2,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug AbuseIntramural Research Program Curated by ChEMBL | Assay Description Displacement of [125]IABN from human D3 receptor expressed in HEK293 cells | J Med Chem 52: 2559-70 (2009) Article DOI: 10.1021/jm900095y BindingDB Entry DOI: 10.7270/Q2HX1DKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50129425 (5-Fluoro-1H-indole-2-carboxylic acid {4-[4-(2,3-di...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Binding affinity towards rat Dopamine receptor D3 in Sf9 cells expressing in recombinant baculovirus (Bv) using [125I]-IABN as radioligand | Bioorg Med Chem Lett 13: 2179-83 (2003) BindingDB Entry DOI: 10.7270/Q21Z43SN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50129425 (5-Fluoro-1H-indole-2-carboxylic acid {4-[4-(2,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Agonistic activity of quinpirole stimulation of mitogenesis in human Dopamine receptor D3 transfected CHO cells | Bioorg Med Chem Lett 13: 2179-83 (2003) BindingDB Entry DOI: 10.7270/Q21Z43SN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50129425 (5-Fluoro-1H-indole-2-carboxylic acid {4-[4-(2,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse- Intramural Research Program Curated by ChEMBL | Assay Description Antagonist activity at human dopamine D3 receptor expressed in CHO cells assessed as inhibition of quinpirole-stimulated mitogenesis | J Med Chem 58: 6195-213 (2015) Article DOI: 10.1021/acs.jmedchem.5b00776 BindingDB Entry DOI: 10.7270/Q2KS6T92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50129425 (5-Fluoro-1H-indole-2-carboxylic acid {4-[4-(2,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Agonistic activity of quinpirole stimulation of mitogenesis in human Dopamine receptor D3 transfected CHO cells | Bioorg Med Chem Lett 13: 2179-83 (2003) BindingDB Entry DOI: 10.7270/Q21Z43SN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50129425 (5-Fluoro-1H-indole-2-carboxylic acid {4-[4-(2,3-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse- Intramural Research Program Curated by ChEMBL | Assay Description Agonist activity at human dopamine D3 receptor expressed in CHO cells assessed as stimulation of quinpirole-stimulated mitogenesis | J Med Chem 58: 6195-213 (2015) Article DOI: 10.1021/acs.jmedchem.5b00776 BindingDB Entry DOI: 10.7270/Q2KS6T92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||