

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3583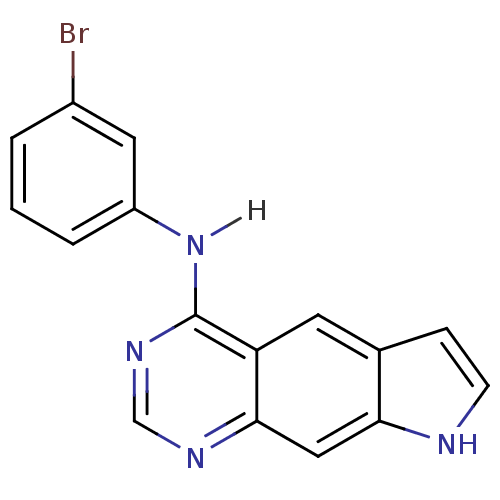 (5-[(3-Bromophenyl)amino]-1H-pyrrolo[3,2-g]quinazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 42: 5464-74 (1999) Article DOI: 10.1021/jm9903949 BindingDB Entry DOI: 10.7270/Q28K7783 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3583 (5-[(3-Bromophenyl)amino]-1H-pyrrolo[3,2-g]quinazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 39: 918-28 (1996) Article DOI: 10.1021/jm950692f BindingDB Entry DOI: 10.7270/Q2222RZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3583 (5-[(3-Bromophenyl)amino]-1H-pyrrolo[3,2-g]quinazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 1519-29 (1997) Article DOI: 10.1021/jm960789h BindingDB Entry DOI: 10.7270/Q2SJ1HRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3583 (5-[(3-Bromophenyl)amino]-1H-pyrrolo[3,2-g]quinazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition autophosphorylation of EGFR in human DiFi cells after 2 hrs by ELISA | Eur J Med Chem 43: 781-91 (2008) Article DOI: 10.1016/j.ejmech.2007.06.006 BindingDB Entry DOI: 10.7270/Q2DJ5GXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||