

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19204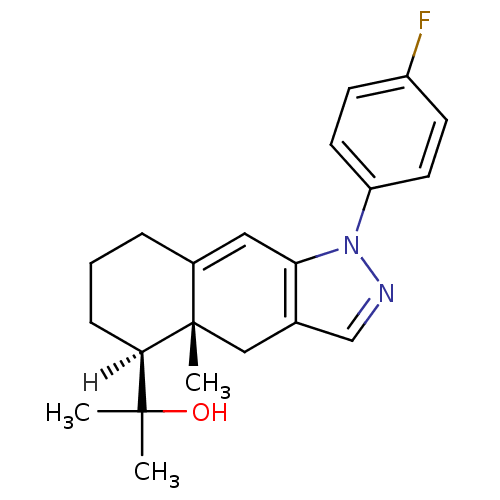 (2-[(4aR,5S)-1-(4-fluorophenyl)-4a-methyl-1H,4H,4aH...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | 3 | n/a | n/a | 7.5 | 4 |
Merck Research Laboratories | Assay Description The IC50s were determined by incubating the receptors with radiolabeled dexamethasone in the presence of full log scale concentrations (10-11 M to 10... | J Med Chem 47: 2441-52 (2004) Article DOI: 10.1021/jm030585i BindingDB Entry DOI: 10.7270/Q28W3BKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM19204 (2-[(4aR,5S)-1-(4-fluorophenyl)-4a-methyl-1H,4H,4aH...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at glucocorticoid receptor in rat INS-1 832/13 cells transfected with CCL2-promoter luciferase plasmid construct assessed as reducti... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00379 BindingDB Entry DOI: 10.7270/Q2VM4H3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM19204 (2-[(4aR,5S)-1-(4-fluorophenyl)-4a-methyl-1H,4H,4aH...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 63 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at glucocorticoid receptor in rat INS-1 832/13 cells transfected with 3XGRE-promoter luciferase plasmid construct assessed as induct... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00379 BindingDB Entry DOI: 10.7270/Q2VM4H3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||