

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50147463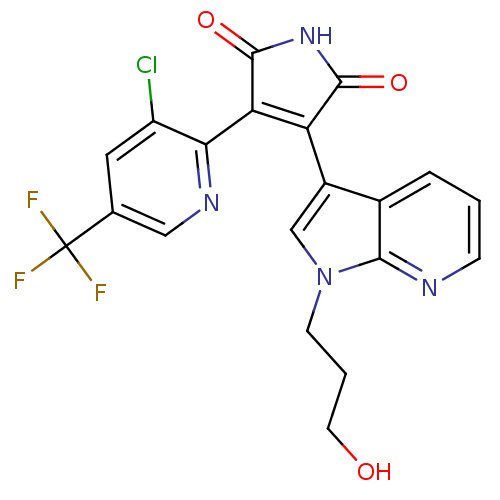 (3-(3-Chloro-5-trifluoromethyl-pyridin-2-yl)-4-[1-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrate | Bioorg Med Chem Lett 14: 3245-50 (2004) Article DOI: 10.1016/j.bmcl.2004.03.090 BindingDB Entry DOI: 10.7270/Q2W958NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50147463 (3-(3-Chloro-5-trifluoromethyl-pyridin-2-yl)-4-[1-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human protein kinase C eta | Eur J Med Chem 164: 448-470 (2019) Article DOI: 10.1016/j.ejmech.2018.12.073 BindingDB Entry DOI: 10.7270/Q2WH2SVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50147463 (3-(3-Chloro-5-trifluoromethyl-pyridin-2-yl)-4-[1-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of GSK-3beta (unknown origin) | Eur J Med Chem 144: 843-858 (2018) Article DOI: 10.1016/j.ejmech.2017.11.103 BindingDB Entry DOI: 10.7270/Q2Q242SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50147463 (3-(3-Chloro-5-trifluoromethyl-pyridin-2-yl)-4-[1-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 620 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Effective concentration of compound against glycogen synthase kinase-3 in HEK293 cells | Bioorg Med Chem Lett 14: 3245-50 (2004) Article DOI: 10.1016/j.bmcl.2004.03.090 BindingDB Entry DOI: 10.7270/Q2W958NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||