

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50446393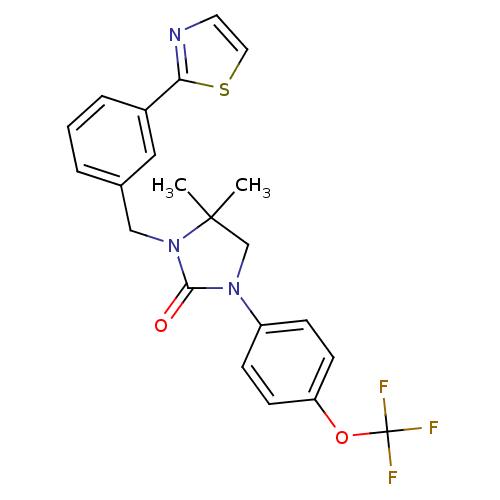 (CHEMBL3109644 | US9181261, 74) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a |
Therachem Research Medilab (India) Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of TrkA (unknown origin) using fluorescently labeled peptide substrate after 3 hrs | ACS Med Chem Lett 5: 8-9 (2014) Article DOI: 10.1021/ml4005073 BindingDB Entry DOI: 10.7270/Q2B56M6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50446393 (CHEMBL3109644 | US9181261, 74) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description TrkA kinase activity was measured as the ability of the enzyme to phosphorylate a fluorescently labeled peptide substrate. Buffer salts, reagents, an... | US Patent US9181261 (2015) BindingDB Entry DOI: 10.7270/Q24F1PJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||