

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50127605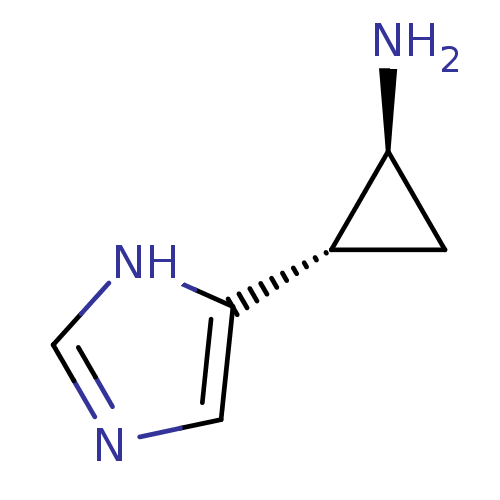 ((1S,2S)-2-(1H-Imidazol-4-yl)-cyclopropylamine | (1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description In vitro binding affinity was measured against Histamine H3 receptor on rat cerebral cortex. | J Med Chem 42: 1115-22 (1999) Article DOI: 10.1021/jm9810912 BindingDB Entry DOI: 10.7270/Q24Q7XPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50127605 ((1S,2S)-2-(1H-Imidazol-4-yl)-cyclopropylamine | (1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Affinity against rat Histamine H3 receptor using [3H]-N-alpha-methyl histamine | J Med Chem 46: 1980-8 (2003) Article DOI: 10.1021/jm020415q BindingDB Entry DOI: 10.7270/Q2XG9QG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50127605 ((1S,2S)-2-(1H-Imidazol-4-yl)-cyclopropylamine | (1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibitory activity measured by [3H]- N alpha- methyl-histamine binding to membranes of SK-N-MC cells expressing the human Histamine H3 receptor | J Med Chem 46: 5445-57 (2003) Article DOI: 10.1021/jm030905y BindingDB Entry DOI: 10.7270/Q2FN18X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50127605 ((1S,2S)-2-(1H-Imidazol-4-yl)-cyclopropylamine | (1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kuopio Curated by ChEMBL | Assay Description Binding affinity of compound against Histamine H3 receptor | J Med Chem 42: 1193-202 (1999) Article DOI: 10.1021/jm980408v BindingDB Entry DOI: 10.7270/Q20Z760D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50127605 ((1S,2S)-2-(1H-Imidazol-4-yl)-cyclopropylamine | (1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Inhibition of human Histamine H3 receptor using [3H]-N-alpha-methyl histamine | J Med Chem 46: 1980-8 (2003) Article DOI: 10.1021/jm020415q BindingDB Entry DOI: 10.7270/Q2XG9QG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50127605 ((1S,2S)-2-(1H-Imidazol-4-yl)-cyclopropylamine | (1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibitory activity determined by the inhibition of cAMP- stimulated beta-galactosidase transcription in SK-N-MC cells expressing the human Histamine... | J Med Chem 46: 5445-57 (2003) Article DOI: 10.1021/jm030905y BindingDB Entry DOI: 10.7270/Q2FN18X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||