

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50179335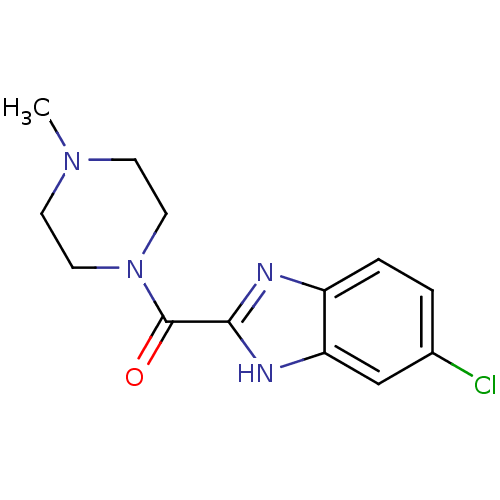 ((5-Chloro-1H-benzoimidazol-2-yl)-(4-methyl-piperaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development, LLC Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in SK-N-MC cells after 45 mins by competition binding analysis | J Med Chem 57: 2429-39 (2014) Article DOI: 10.1021/jm401727m BindingDB Entry DOI: 10.7270/Q25H7HSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50179335 ((5-Chloro-1H-benzoimidazol-2-yl)-(4-methyl-piperaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from recombinant human histamine H4 receptor in SK-N-MC cells | J Med Chem 48: 8289-98 (2005) Article DOI: 10.1021/jm0502081 BindingDB Entry DOI: 10.7270/Q2C24W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50179335 ((5-Chloro-1H-benzoimidazol-2-yl)-(4-methyl-piperaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor | Bioorg Med Chem Lett 21: 3113-6 (2011) Article DOI: 10.1016/j.bmcl.2011.03.017 BindingDB Entry DOI: 10.7270/Q24X5845 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50179335 ((5-Chloro-1H-benzoimidazol-2-yl)-(4-methyl-piperaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffin Discoveries BV Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human H4 receptor expressed in HEK cell membranes | Bioorg Med Chem Lett 22: 461-7 (2011) Article DOI: 10.1016/j.bmcl.2011.10.104 BindingDB Entry DOI: 10.7270/Q2D79BT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50179335 ((5-Chloro-1H-benzoimidazol-2-yl)-(4-methyl-piperaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 39.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H4 receptor expressed in HEK293 cells assessed as rev inhibition of forskolin-stimulated cAMP accumulation by ... | Bioorg Med Chem Lett 22: 1156-9 (2012) Article DOI: 10.1016/j.bmcl.2011.11.098 BindingDB Entry DOI: 10.7270/Q2Q81DJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50179335 ((5-Chloro-1H-benzoimidazol-2-yl)-(4-methyl-piperaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 39.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H4 receptor by functional assay | Bioorg Med Chem Lett 21: 6591-5 (2011) Article DOI: 10.1016/j.bmcl.2011.07.114 BindingDB Entry DOI: 10.7270/Q2DF6RNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50179335 ((5-Chloro-1H-benzoimidazol-2-yl)-(4-methyl-piperaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 47.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in CHO cells coexpressing Ga15 by radioligand filtration binding... | Bioorg Med Chem Lett 22: 1156-9 (2012) Article DOI: 10.1016/j.bmcl.2011.11.098 BindingDB Entry DOI: 10.7270/Q2Q81DJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50179335 ((5-Chloro-1H-benzoimidazol-2-yl)-(4-methyl-piperaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 47.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique | Bioorg Med Chem Lett 21: 6591-5 (2011) Article DOI: 10.1016/j.bmcl.2011.07.114 BindingDB Entry DOI: 10.7270/Q2DF6RNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||