

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50105680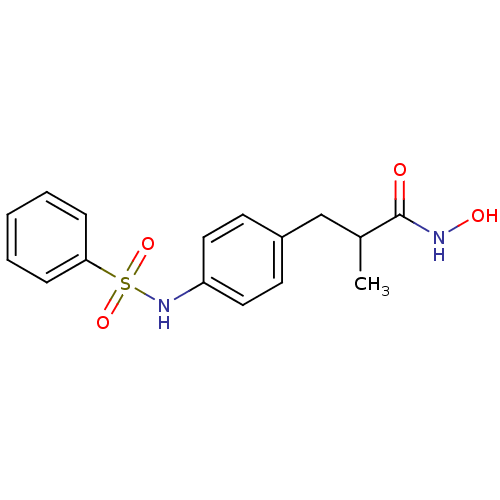 (3-(4-Benzenesulfonylamino-phenyl)-N-hydroxy-2-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Methylgene Inc. US Patent | Assay Description For deacetylase assays, 20,000 cpm of the [3H]-metabolically labeled acetylated histone substrate (M. Yoshida et al., J. Biol. Chem. 265(28): 17174-1... | US Patent US8796330 (2014) BindingDB Entry DOI: 10.7270/Q22B8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50105680 (3-(4-Benzenesulfonylamino-phenyl)-N-hydroxy-2-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description In vitro inhibition of partially purified recombinant human Histone deacetylase 1 | Bioorg Med Chem Lett 11: 2847-50 (2001) BindingDB Entry DOI: 10.7270/Q2SF2VF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50105680 (3-(4-Benzenesulfonylamino-phenyl)-N-hydroxy-2-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aton Pharma, Inc Curated by ChEMBL | Assay Description Inhibitory concentration against human Histone deacetylase 1 | J Med Chem 46: 5097-116 (2003) Article DOI: 10.1021/jm0303094 BindingDB Entry DOI: 10.7270/Q2MP5413 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50105680 (3-(4-Benzenesulfonylamino-phenyl)-N-hydroxy-2-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a |
MethylGene Inc. Curated by ChEMBL | Assay Description Concentration of compound required for acetylation of histone-4 in human T24 cancer cells | Bioorg Med Chem Lett 11: 2847-50 (2001) BindingDB Entry DOI: 10.7270/Q2SF2VF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||