

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 7 (Homo sapiens (Human)) | BDBM50397360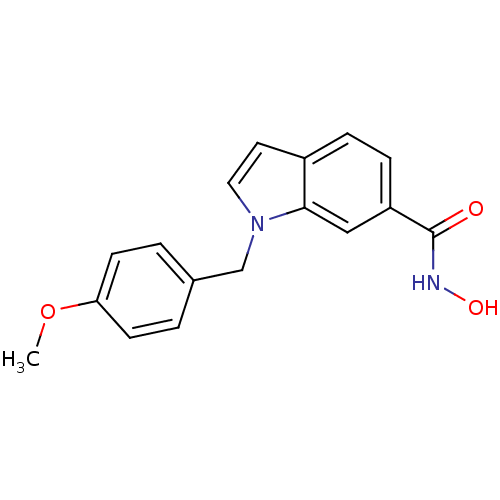 (CHEMBL2170177 | US10188756, Compound CN110) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
SynBioC Research Group, Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, B-9000 Ghent, Belgium. Curated by ChEMBL | Assay Description Inhibition of HDAC7 (unknown origin) using acetyl-Gly-Ala-(N-acetyl-Lys)-amino-4-methylcoumarin as substrate after 15 mins by fluorescence assay | Eur J Med Chem 135: 174-195 (2017) Article DOI: 10.1016/j.ejmech.2017.04.013 BindingDB Entry DOI: 10.7270/Q2G44SQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 7 (Homo sapiens (Human)) | BDBM50397360 (CHEMBL2170177 | US10188756, Compound CN110) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Mississauga Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human HDAC7 (518 to end residues) expressed in baculovirus expression system using FAM-RHKK(TF-Ac)-NH2 substrate ... | J Med Chem 63: 8634-8648 (2020) Article DOI: 10.1021/acs.jmedchem.0c01025 BindingDB Entry DOI: 10.7270/Q2CC1470 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 7 (Homo sapiens (Human)) | BDBM50397360 (CHEMBL2170177 | US10188756, Compound CN110) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | US Patent | n/a | n/a | >7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation US Patent | Assay Description All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... | US Patent US10188756 (2019) BindingDB Entry DOI: 10.7270/Q20Z75CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||