

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50089657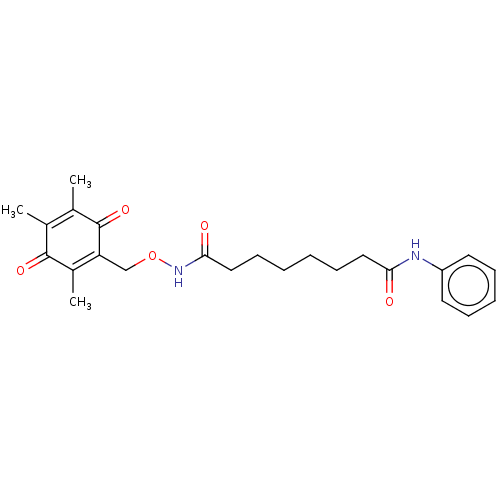 (CHEMBL3577298) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Binding affinity to human Zn2+-HDAC8 assessed as loss of activity by Fluor-de-Lys activity assay | J Med Chem 58: 4812-21 (2015) Article DOI: 10.1021/acs.jmedchem.5b00539 BindingDB Entry DOI: 10.7270/Q2HH6MTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50089657 (CHEMBL3577298) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Reversible-time dependent inhibition of human wild type HDAC8 by Fluor-de-Lys activity assay | J Med Chem 58: 4812-21 (2015) Article DOI: 10.1021/acs.jmedchem.5b00539 BindingDB Entry DOI: 10.7270/Q2HH6MTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50089657 (CHEMBL3577298) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC8 using MAZ1675 as fluorogenic substrate measured every 5 mins by optimized homogenous fluorescence based assay | J Med Chem 58: 4812-21 (2015) Article DOI: 10.1021/acs.jmedchem.5b00539 BindingDB Entry DOI: 10.7270/Q2HH6MTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||