 Found 8 hits Enz. Inhib. hit(s) with Target = 'Histone-lysine N-methyltransferase EZH2' and Ligand = 'BDBM50010823'
Found 8 hits Enz. Inhib. hit(s) with Target = 'Histone-lysine N-methyltransferase EZH2' and Ligand = 'BDBM50010823' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010823
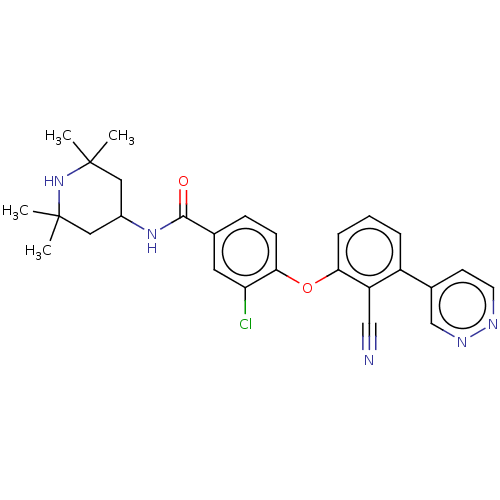
(CHEMBL3264787)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2cccc(-c3ccnnc3)c2C#N)c(Cl)c1 Show InChI InChI=1S/C27H28ClN5O2/c1-26(2)13-19(14-27(3,4)33-26)32-25(34)17-8-9-24(22(28)12-17)35-23-7-5-6-20(21(23)15-29)18-10-11-30-31-16-18/h5-12,16,19,33H,13-14H2,1-4H3,(H,32,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01208
BindingDB Entry DOI: 10.7270/Q25X2DZR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010823

(CHEMBL3264787)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2cccc(-c3ccnnc3)c2C#N)c(Cl)c1 Show InChI InChI=1S/C27H28ClN5O2/c1-26(2)13-19(14-27(3,4)33-26)32-25(34)17-8-9-24(22(28)12-17)35-23-7-5-6-20(21(23)15-29)18-10-11-30-31-16-18/h5-12,16,19,33H,13-14H2,1-4H3,(H,32,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of wild type EZH2 (unknown origin) |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010823

(CHEMBL3264787)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2cccc(-c3ccnnc3)c2C#N)c(Cl)c1 Show InChI InChI=1S/C27H28ClN5O2/c1-26(2)13-19(14-27(3,4)33-26)32-25(34)17-8-9-24(22(28)12-17)35-23-7-5-6-20(21(23)15-29)18-10-11-30-31-16-18/h5-12,16,19,33H,13-14H2,1-4H3,(H,32,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count based method |
Bioorg Med Chem Lett 25: 3644-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.056
BindingDB Entry DOI: 10.7270/Q2Q24205 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010823

(CHEMBL3264787)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2cccc(-c3ccnnc3)c2C#N)c(Cl)c1 Show InChI InChI=1S/C27H28ClN5O2/c1-26(2)13-19(14-27(3,4)33-26)32-25(34)17-8-9-24(22(28)12-17)35-23-7-5-6-20(21(23)15-29)18-10-11-30-31-16-18/h5-12,16,19,33H,13-14H2,1-4H3,(H,32,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00047
BindingDB Entry DOI: 10.7270/Q27D3059 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010823

(CHEMBL3264787)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2cccc(-c3ccnnc3)c2C#N)c(Cl)c1 Show InChI InChI=1S/C27H28ClN5O2/c1-26(2)13-19(14-27(3,4)33-26)32-25(34)17-8-9-24(22(28)12-17)35-23-7-5-6-20(21(23)15-29)18-10-11-30-31-16-18/h5-12,16,19,33H,13-14H2,1-4H3,(H,32,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as global reduction in H3K27me3 level after 10 days by ELISA-based assay |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010823

(CHEMBL3264787)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2cccc(-c3ccnnc3)c2C#N)c(Cl)c1 Show InChI InChI=1S/C27H28ClN5O2/c1-26(2)13-19(14-27(3,4)33-26)32-25(34)17-8-9-24(22(28)12-17)35-23-7-5-6-20(21(23)15-29)18-10-11-30-31-16-18/h5-12,16,19,33H,13-14H2,1-4H3,(H,32,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as global reduction in H3K27me3 level after 7 days by ELISA-based assay |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010823

(CHEMBL3264787)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2cccc(-c3ccnnc3)c2C#N)c(Cl)c1 Show InChI InChI=1S/C27H28ClN5O2/c1-26(2)13-19(14-27(3,4)33-26)32-25(34)17-8-9-24(22(28)12-17)35-23-7-5-6-20(21(23)15-29)18-10-11-30-31-16-18/h5-12,16,19,33H,13-14H2,1-4H3,(H,32,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human KARPAS422 cells assessed as global reduction in H3K27me3 level after 4 days by ELISA-based assay |
ACS Med Chem Lett 5: 378-83 (2014)
Article DOI: 10.1021/ml400494b
BindingDB Entry DOI: 10.7270/Q2BP04BR |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50010823

(CHEMBL3264787)Show SMILES CC1(C)CC(CC(C)(C)N1)NC(=O)c1ccc(Oc2cccc(-c3ccnnc3)c2C#N)c(Cl)c1 Show InChI InChI=1S/C27H28ClN5O2/c1-26(2)13-19(14-27(3,4)33-26)32-25(34)17-8-9-24(22(28)12-17)35-23-7-5-6-20(21(23)15-29)18-10-11-30-31-16-18/h5-12,16,19,33H,13-14H2,1-4H3,(H,32,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 in human HeLa cells assessed as reduction of H3K27me3 and H3K27me2 level after 96 hrs by ELISA method |
J Med Chem 58: 1596-629 (2015)
Article DOI: 10.1021/jm501234a
BindingDB Entry DOI: 10.7270/Q28K7BS2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data