 Found 6 hits Enz. Inhib. hit(s) with Target = 'Histone-lysine N-methyltransferase EZH2' and Ligand = 'BDBM50400783'
Found 6 hits Enz. Inhib. hit(s) with Target = 'Histone-lysine N-methyltransferase EZH2' and Ligand = 'BDBM50400783' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50400783
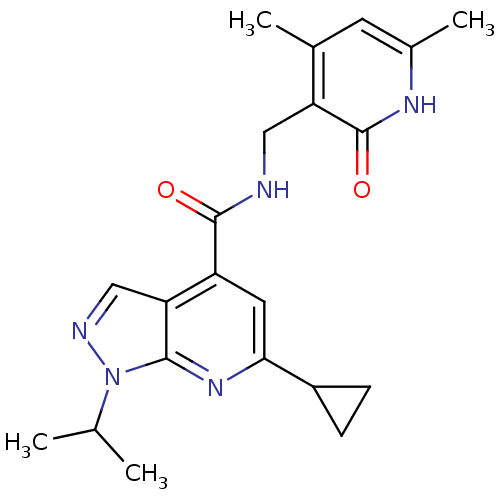
(CHEMBL1608462)Show SMILES CC(C)n1ncc2c(cc(nc12)C1CC1)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C21H25N5O2/c1-11(2)26-19-17(10-23-26)15(8-18(25-19)14-5-6-14)20(27)22-9-16-12(3)7-13(4)24-21(16)28/h7-8,10-11,14H,5-6,9H2,1-4H3,(H,22,27)(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
| Assay Description
Competitive inhibition of human EZH2 using H3K27A as substrate in presence of SAM |
J Med Chem 58: 1596-629 (2015)
Article DOI: 10.1021/jm501234a
BindingDB Entry DOI: 10.7270/Q28K7BS2 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50400783

(CHEMBL1608462)Show SMILES CC(C)n1ncc2c(cc(nc12)C1CC1)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C21H25N5O2/c1-11(2)26-19-17(10-23-26)15(8-18(25-19)14-5-6-14)20(27)22-9-16-12(3)7-13(4)24-21(16)28/h7-8,10-11,14H,5-6,9H2,1-4H3,(H,22,27)(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of human EZH2 using [3H]-SAM as substrate in presence of H3K27A |
J Med Chem 58: 1596-629 (2015)
Article DOI: 10.1021/jm501234a
BindingDB Entry DOI: 10.7270/Q28K7BS2 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50400783

(CHEMBL1608462)Show SMILES CC(C)n1ncc2c(cc(nc12)C1CC1)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C21H25N5O2/c1-11(2)26-19-17(10-23-26)15(8-18(25-19)14-5-6-14)20(27)22-9-16-12(3)7-13(4)24-21(16)28/h7-8,10-11,14H,5-6,9H2,1-4H3,(H,22,27)(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human FLAG-tev-fused EZH2 expressed in baculovirus infected Sf9 insect cells using H3K27 peptide as substrate in presence of [3H]-SAM b... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01344
BindingDB Entry DOI: 10.7270/Q23R0XMC |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50400783

(CHEMBL1608462)Show SMILES CC(C)n1ncc2c(cc(nc12)C1CC1)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C21H25N5O2/c1-11(2)26-19-17(10-23-26)15(8-18(25-19)14-5-6-14)20(27)22-9-16-12(3)7-13(4)24-21(16)28/h7-8,10-11,14H,5-6,9H2,1-4H3,(H,22,27)(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00047
BindingDB Entry DOI: 10.7270/Q27D3059 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50400783

(CHEMBL1608462)Show SMILES CC(C)n1ncc2c(cc(nc12)C1CC1)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C21H25N5O2/c1-11(2)26-19-17(10-23-26)15(8-18(25-19)14-5-6-14)20(27)22-9-16-12(3)7-13(4)24-21(16)28/h7-8,10-11,14H,5-6,9H2,1-4H3,(H,22,27)(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human EZH2 (2 to 746 residues) expressed in baculovirus infected insect cells using histone H3 as substrate preincubated for 10 mins fo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01344
BindingDB Entry DOI: 10.7270/Q23R0XMC |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50400783

(CHEMBL1608462)Show SMILES CC(C)n1ncc2c(cc(nc12)C1CC1)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C21H25N5O2/c1-11(2)26-19-17(10-23-26)15(8-18(25-19)14-5-6-14)20(27)22-9-16-12(3)7-13(4)24-21(16)28/h7-8,10-11,14H,5-6,9H2,1-4H3,(H,22,27)(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of EZH2-mediated proliferation of human LNCaP cells after 6 days by chemiluminescence analysis |
ACS Med Chem Lett 3: 1091-1096 (2012)
Article DOI: 10.1021/ml3003346
BindingDB Entry DOI: 10.7270/Q2NK3G60 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data