

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ileal sodium/bile acid cotransporter (Mus musculus) | BDBM50434849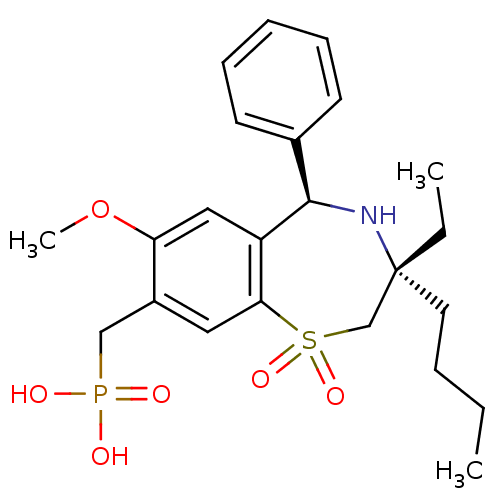 (CHEMBL2385105 | US9040518, 6) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of mouse ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Rattus norvegicus) | BDBM50434849 (CHEMBL2385105 | US9040518, 6) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of rat ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysis | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434849 (CHEMBL2385105 | US9040518, 6) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | 25 |
GlaxoSmithKline LLC US Patent | Assay Description On the day of the uptake experiment, 10 mM HEPES was added to Hank's Balanced Salt Solution, and the pH was adjusted to 7.4 with TRIS (HBSSH). The as... | US Patent US9040518 (2015) BindingDB Entry DOI: 10.7270/Q2T72G58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ileal sodium/bile acid cotransporter (Homo sapiens (Human)) | BDBM50434849 (CHEMBL2385105 | US9040518, 6) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research& Development Curated by ChEMBL | Assay Description Inhibition of human ASBT expressed in HEK293 cells assessed as inhibition of [3H]-taurocholate uptake after 90 mins by scintillation counting analysi... | J Med Chem 56: 5094-114 (2013) Article DOI: 10.1021/jm400459m BindingDB Entry DOI: 10.7270/Q2MC91D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||