

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50051818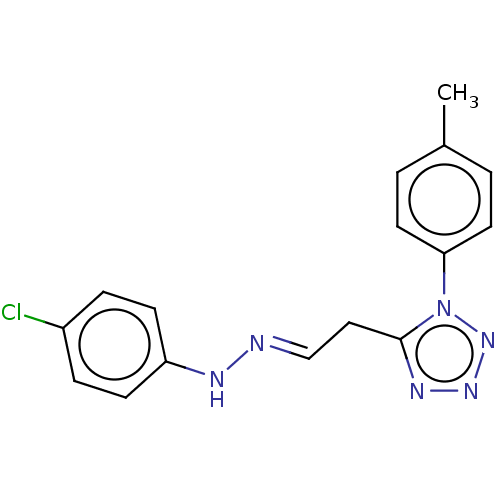 (CHEMBL3318333) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 expressed in mouse P815B cells using L-tryptophan substrate incubated for 18 hrs by HPLC based cellular assay | Eur J Med Chem 84: 284-301 (2014) Article DOI: 10.1016/j.ejmech.2014.06.078 BindingDB Entry DOI: 10.7270/Q21C1ZJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50051818 (CHEMBL3318333) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 5 mM GSH by HPLC | Eur J Med Chem 84: 284-301 (2014) Article DOI: 10.1016/j.ejmech.2014.06.078 BindingDB Entry DOI: 10.7270/Q21C1ZJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50051818 (CHEMBL3318333) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins in presence of 0.01% Triton-X by HPLC | Eur J Med Chem 84: 284-301 (2014) Article DOI: 10.1016/j.ejmech.2014.06.078 BindingDB Entry DOI: 10.7270/Q21C1ZJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50051818 (CHEMBL3318333) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLC | Eur J Med Chem 84: 284-301 (2014) Article DOI: 10.1016/j.ejmech.2014.06.078 BindingDB Entry DOI: 10.7270/Q21C1ZJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||