

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303910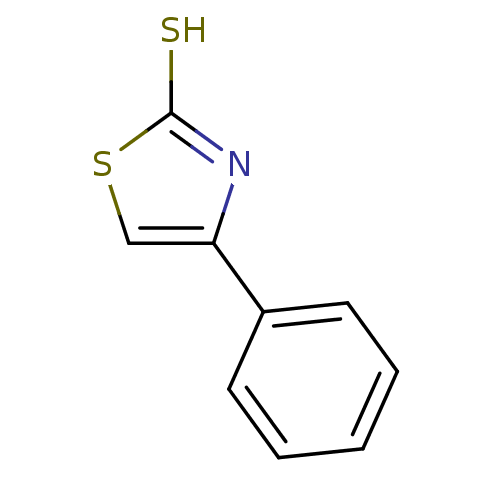 (4-phenylthiazole-2-thiol | CHEMBL571436) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in HEK293 cells assessed as blockade of tryptophan degradation by HPLC | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50303910 (4-phenylthiazole-2-thiol | CHEMBL571436) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of mouse recombinant IDO expressed in mouse P815B cells assessed as blockade of tryptophan degradation by HPLC | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303910 (4-phenylthiazole-2-thiol | CHEMBL571436) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NAMEDIC-NARILIS, University of Namur, 61 Rue de Bruxelles, 5000 Namur, Belgium; Laboratory of Organic Synthesis and Heterocyclic Chemistry, Department of Chemistry, College of Sciences at Tunis, El M Curated by ChEMBL | Assay Description Inhibition of human IDO1 pre-incubated for 10 mins before L-Trp as substrate addition and measured after 30 mins by colorimetry | Bioorg Med Chem Lett 27: 3607-3610 (2017) Article DOI: 10.1016/j.bmcl.2016.06.052 BindingDB Entry DOI: 10.7270/Q2F1925G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303910 (4-phenylthiazole-2-thiol | CHEMBL571436) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NAMEDIC-NARILIS, University of Namur, 61 Rue de Bruxelles, 5000 Namur, Belgium; Laboratory of Organic Synthesis and Heterocyclic Chemistry, Department of Chemistry, College of Sciences at Tunis, El M Curated by ChEMBL | Assay Description Inhibition of human IDO1 pre-incubated for 10 mins before L-Trp as substrate addition and measured after 30 mins by colorimetry | Bioorg Med Chem Lett 27: 3607-3610 (2017) Article DOI: 10.1016/j.bmcl.2016.06.052 BindingDB Entry DOI: 10.7270/Q2F1925G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303910 (4-phenylthiazole-2-thiol | CHEMBL571436) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli BL21 AI | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||