

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239489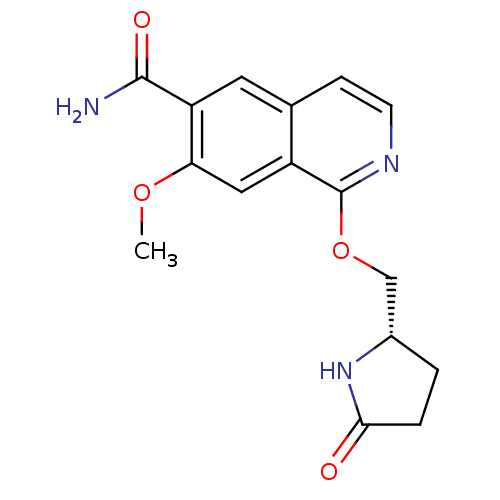 (CHEMBL4100091 | US10329302, Example 121 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital | Assay Description This is an in vitro assay to measure IRAK4 enzymatic activity utilizing the DELFIA (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, Perkin-... | ACS Chem Biol 4: 834-43 (2009) BindingDB Entry DOI: 10.7270/Q2FT8PC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239489 (CHEMBL4100091 | US10329302, Example 121 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... | US Patent US10793579 (2020) BindingDB Entry DOI: 10.7270/Q2H9988P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239489 (CHEMBL4100091 | US10329302, Example 121 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human full length IRAK4 preincubated for 20 mins followed by biotinylated-AGAGRDKYKTLRQIR substrate addition in ... | J Med Chem 60: 5521-5542 (2017) Article DOI: 10.1021/acs.jmedchem.7b00231 BindingDB Entry DOI: 10.7270/Q26D5W42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239489 (CHEMBL4100091 | US10329302, Example 121 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27D308N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239489 (CHEMBL4100091 | US10329302, Example 121 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Protocol B: To begin the assay, 45 μL of reaction mixture containing 20 mM HEPES pH=7.5, 5 mM MgCl2, 0.0025% Brij-35, 600 μM ATP, 228 μ... | US Patent US10793579 (2020) BindingDB Entry DOI: 10.7270/Q2H9988P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50239489 (CHEMBL4100091 | US10329302, Example 121 | US107935...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 347 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of IRAK4 in human PBMC assessed as reduction in R848-stimulated TNF alpha production after 3 hrs | J Med Chem 60: 5521-5542 (2017) Article DOI: 10.1021/acs.jmedchem.7b00231 BindingDB Entry DOI: 10.7270/Q26D5W42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||