

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM21865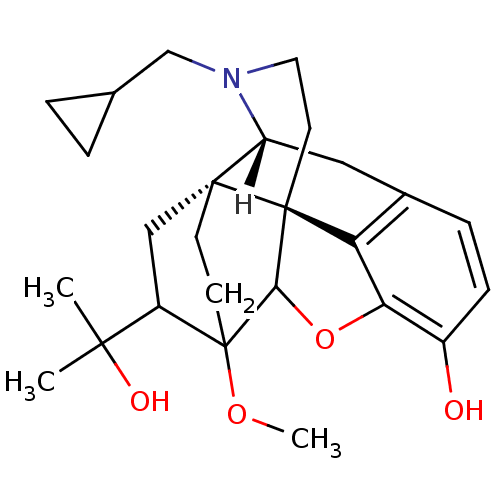 ((14beta)-17-(cyclopropylmethyl)-18-(1-hydroxy-1-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center Curated by PDSP Ki Database | J Pharmacol Exp Ther 270: 1246-55 (1994) BindingDB Entry DOI: 10.7270/Q2CF9NN9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM21865 ((14beta)-17-(cyclopropylmethyl)-18-(1-hydroxy-1-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDA Addiction Research Center Curated by PDSP Ki Database | Life Sci 45: 1821-9 (1989) Article DOI: 10.1016/0024-3205(89)90523-7 BindingDB Entry DOI: 10.7270/Q20Z71SN | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||