

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50105085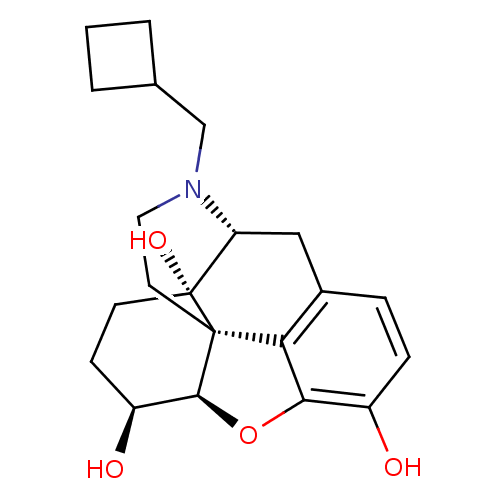 (17-cyclobutylmethyl-4,5alpha-epoxymorphinan-3,6alp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from human opioid kappa receptor expressed in CHO cells | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50105085 (17-cyclobutylmethyl-4,5alpha-epoxymorphinan-3,6alp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 19: 2289-94 (2009) Article DOI: 10.1016/j.bmcl.2009.02.078 BindingDB Entry DOI: 10.7270/Q200030V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50105085 (17-cyclobutylmethyl-4,5alpha-epoxymorphinan-3,6alp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by PDSP Ki Database | Mol Pharmacol 45: 330-4 (1994) Article DOI: 10.1016/j.bioorg.2015.02.008 BindingDB Entry DOI: 10.7270/Q21Z42X1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50105085 (17-cyclobutylmethyl-4,5alpha-epoxymorphinan-3,6alp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank US Patent | 25.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inheris Pharmaceuticals, Inc. US Patent | Assay Description Briefly, the receptor binding affinity of the nalbuphine and PEG-nalbuphine conjugates was measured using radioligand binding assays in CHO cells tha... | US Patent US10512644 (2019) BindingDB Entry DOI: 10.7270/Q2RN3B8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50105085 (17-cyclobutylmethyl-4,5alpha-epoxymorphinan-3,6alp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank US Patent | 29.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nektar Therapeutics US Patent | Assay Description Specific binding is determined by subtraction of the cpm bound in the presence of 50-100× excess of cold ligand. Binding data assays were analyzed us... | US Patent US9233167 (2016) BindingDB Entry DOI: 10.7270/Q25T3J97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50105085 (17-cyclobutylmethyl-4,5alpha-epoxymorphinan-3,6alp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 using [3H]- U-69,593 radioligand | J Med Chem 44: 3378-90 (2001) BindingDB Entry DOI: 10.7270/Q23X87B8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50105085 (17-cyclobutylmethyl-4,5alpha-epoxymorphinan-3,6alp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human opioid kappa receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 50: 2254-8 (2007) Article DOI: 10.1021/jm061327z BindingDB Entry DOI: 10.7270/Q2CZ3804 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50105085 (17-cyclobutylmethyl-4,5alpha-epoxymorphinan-3,6alp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 250 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human KOPR expressed in U2OS cells assessed as beta-arrestin2 recruitment after 90 mins by DiscoveRx PathHunter assay | Bioorg Med Chem Lett 22: 1023-6 (2012) Article DOI: 10.1016/j.bmcl.2011.11.128 BindingDB Entry DOI: 10.7270/Q2S182X2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50105085 (17-cyclobutylmethyl-4,5alpha-epoxymorphinan-3,6alp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 56 | n/a | n/a | n/a | n/a |
Rensselaer Polytechnic Institute Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | Bioorg Med Chem Lett 19: 2289-94 (2009) Article DOI: 10.1016/j.bmcl.2009.02.078 BindingDB Entry DOI: 10.7270/Q200030V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50105085 (17-cyclobutylmethyl-4,5alpha-epoxymorphinan-3,6alp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank US Patent | n/a | n/a | n/a | n/a | 25.1 | n/a | n/a | 7.4 | 30 |
Nektar Therapeutics US Patent | Assay Description Test compound and/or vehicle was preincubated with the cell membranes and 3 uM GDP in modified HEPES buffer (pH 7.4) for 20 minutes, followed by addi... | US Patent US9233167 (2016) BindingDB Entry DOI: 10.7270/Q25T3J97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50105085 (17-cyclobutylmethyl-4,5alpha-epoxymorphinan-3,6alp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 65 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human KOPR expressed in CHO cells assessed as [35S]GTPgammaS binding after 60 mins by liquid scintillation counting | Bioorg Med Chem Lett 22: 1023-6 (2012) Article DOI: 10.1016/j.bmcl.2011.11.128 BindingDB Entry DOI: 10.7270/Q2S182X2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||