

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM283199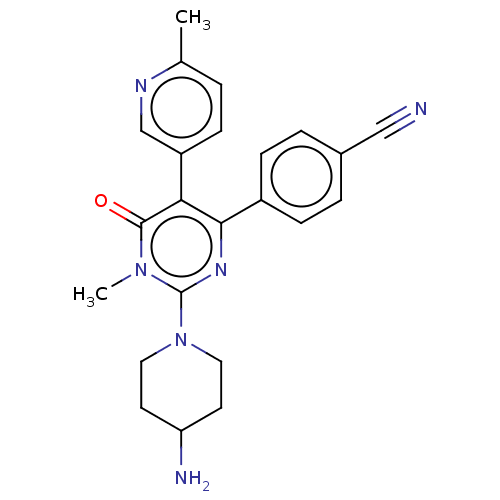 (4-[2-(4-amino-piperidin-1-yl)-1- methyl-5-(6-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | 25 |
CELGENE QUANTICEL RESEARCH, INC. US Patent | Assay Description This assay determines the ability of a test compound to inhibit LSD1 demethylase activity. E. coli expressed full-length human LSD1 (Accession number... | US Patent US10023543 (2018) BindingDB Entry DOI: 10.7270/Q2GQ70TM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM283199 (4-[2-(4-amino-piperidin-1-yl)-1- methyl-5-(6-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | 25 |
Celgene Quanticel Research, Inc. US Patent | Assay Description This assay determines the ability of a test compound to inhibit LSD1 demethylase activity. E. coli expressed full-length human LSD1 (Accession number... | US Patent US9573930 (2017) BindingDB Entry DOI: 10.7270/Q2H70HV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM283199 (4-[2-(4-amino-piperidin-1-yl)-1- methyl-5-(6-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc. US Patent | Assay Description The enzymatic assay of LSD1 activity is based on Time Resolved-Fluorescence Resonance Energy Transfer (TR-FRET) detection. The inhibitory properties ... | US Patent US9776974 (2017) BindingDB Entry DOI: 10.7270/Q2BZ686N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM283199 (4-[2-(4-amino-piperidin-1-yl)-1- methyl-5-(6-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc. US Patent | Assay Description The enzymatic assay of LSD1 activity is based on Time Resolved-Fluorescence Resonance Energy Transfer (TR-FRET) detection. The inhibitory properties ... | US Patent US9771329 (2017) BindingDB Entry DOI: 10.7270/Q2NG4SR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM283199 (4-[2-(4-amino-piperidin-1-yl)-1- methyl-5-(6-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description This assay determines the ability of a test compound to inhibit LSD-1 demethylase activity. E. coli expressed full-length human LSD-1 (Accession numb... | Bioorg Med Chem 17: 7324-36 (2009) BindingDB Entry DOI: 10.7270/Q2W66P3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM283199 (4-[2-(4-amino-piperidin-1-yl)-1- methyl-5-(6-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay determines the ability of a test compound to inhibit LSD1 demethylase activity. E. coli expressed full-length human LSD1 (Accession number... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TF01G7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM283199 (4-[2-(4-amino-piperidin-1-yl)-1- methyl-5-(6-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
CELGENE QUANTICEL RESEARCH, INC. US Patent | Assay Description To analyze LSD1 inhibitor efficacy in cells, a CD11b flow cytometry assay was performed. LSD1 inhibition induces CD11b expression in THP-1 (AML) cell... | US Patent US10207999 (2019) BindingDB Entry DOI: 10.7270/Q2DJ5HRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM283199 (4-[2-(4-amino-piperidin-1-yl)-1- methyl-5-(6-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc. US Patent | Assay Description This assay determines the ability of a test compound to inhibit LSD-1 demethylase activity. E. coli expressed full-length human LSD-1 (Accession numb... | US Patent US10543213 (2020) BindingDB Entry DOI: 10.7270/Q2X63QBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM283199 (4-[2-(4-amino-piperidin-1-yl)-1- methyl-5-(6-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Quanticel Research, Inc. US Patent | Assay Description This assay determines the ability of a test compound to inhibit LSD-1 demethylase activity. E. coli expressed full-length human LSD-1 (Accession numb... | US Patent US10548896 (2020) BindingDB Entry DOI: 10.7270/Q29889CV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM283199 (4-[2-(4-amino-piperidin-1-yl)-1- methyl-5-(6-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
CELGENE QUANTICEL RESEARCH, INC. US Patent | Assay Description This assay determines the ability of a test compound to inhibit LSD-1 demethylase activity. E. coli expressed full-length human LSD-1 (Accession numb... | US Patent US10849898 (2020) BindingDB Entry DOI: 10.7270/Q2XS5ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM283199 (4-[2-(4-amino-piperidin-1-yl)-1- methyl-5-(6-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
CELGENE QUANTICEL RESEARCH, INC. US Patent | Assay Description The enzymatic assay of LSD-1 activity is based on Tnne Resolved-Fluorescence Resonance Energy Transfer (TR-FRET) detection. The inhibitory properties... | US Patent US10960005 (2021) BindingDB Entry DOI: 10.7270/Q2CR5XFH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM283199 (4-[2-(4-amino-piperidin-1-yl)-1- methyl-5-(6-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
CELGENE QUANTICEL RESEARCH, INC. US Patent | Assay Description This assay determines the ability of a test compound to inhibit LSD1 demethylase activity. E. coli expressed full-length human LSD1 (Accession number... | US Patent US10207999 (2019) BindingDB Entry DOI: 10.7270/Q2DJ5HRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||