

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysosomal Pro-X carboxypeptidase (Mus musculus) | BDBM50364676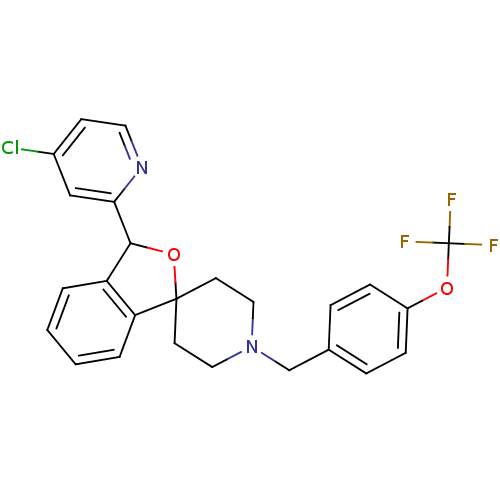 (CHEMBL1951465 | US8785634, 1) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse PrCP | Bioorg Med Chem Lett 22: 1550-6 (2012) Article DOI: 10.1016/j.bmcl.2012.01.002 BindingDB Entry DOI: 10.7270/Q2JS9QXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal Pro-X carboxypeptidase (Homo sapiens (Human)) | BDBM50364676 (CHEMBL1951465 | US8785634, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human PrCP | Bioorg Med Chem Lett 22: 1550-6 (2012) Article DOI: 10.1016/j.bmcl.2012.01.002 BindingDB Entry DOI: 10.7270/Q2JS9QXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal Pro-X carboxypeptidase (Homo sapiens (Human)) | BDBM50364676 (CHEMBL1951465 | US8785634, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp US Patent | Assay Description The potency of compounds of formula I against PrCP was determined by a fluorescence intensity kinetic assay measuring the IC50 values of PrCP inhibit... | US Patent US8785634 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal Pro-X carboxypeptidase (Mus musculus) | BDBM50364676 (CHEMBL1951465 | US8785634, 1) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of PrCP in mouse plasma assessed as angiotensin 3 cleavage by whole serum shift assay | Bioorg Med Chem Lett 22: 1550-6 (2012) Article DOI: 10.1016/j.bmcl.2012.01.002 BindingDB Entry DOI: 10.7270/Q2JS9QXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||