

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM17849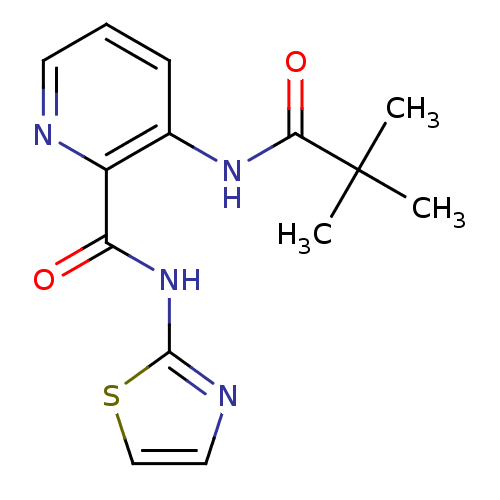 (3-(2,2-dimethylpropanamido)-N-(1,3-thiazol-2-yl)py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM17849 (3-(2,2-dimethylpropanamido)-N-(1,3-thiazol-2-yl)py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Johns Hopkins University | Assay Description The MetAP reaction with Co2+ as a cofactor is coupled to a prolyl aminopeptidase (ProAP) using Met-Pro-p-nitroanilide as substrate. MetAP-catalyzed c... | Proc Natl Acad Sci U S A 103: 18148-53 (2006) Article DOI: 10.1073/pnas.0608389103 BindingDB Entry DOI: 10.7270/Q2RB72VK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||