 Found 10 hits Enz. Inhib. hit(s) with Target = 'Mu-type opioid receptor' and Ligand = 'BDBM50268462'
Found 10 hits Enz. Inhib. hit(s) with Target = 'Mu-type opioid receptor' and Ligand = 'BDBM50268462' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50268462
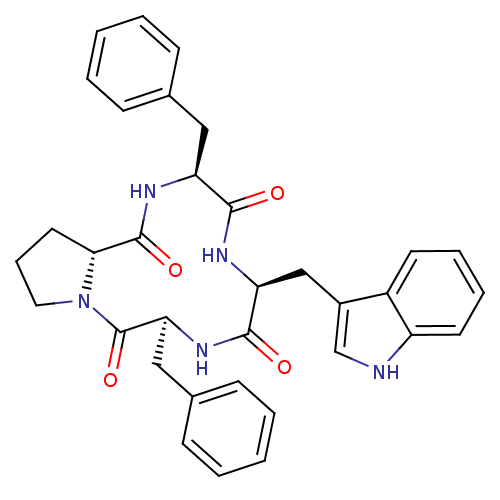
(CHEMBL506616 | c[L-Phe-D-pro-L-Phe-L-trp])Show SMILES O=C1N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCC[C@H]12 |r| Show InChI InChI=1S/C34H35N5O4/c40-31-27(18-22-10-3-1-4-11-22)37-33(42)30-16-9-17-39(30)34(43)29(19-23-12-5-2-6-13-23)38-32(41)28(36-31)20-24-21-35-26-15-8-7-14-25(24)26/h1-8,10-15,21,27-30,35H,9,16-20H2,(H,36,40)(H,37,42)(H,38,41)/t27-,28-,29-,30+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from human MOR expressed in HEK293 cell membranes incubated for 90 mins by liquid scintillation counting |
J Med Chem 59: 9255-9261 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00420
BindingDB Entry DOI: 10.7270/Q2JS9TXZ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50268462

(CHEMBL506616 | c[L-Phe-D-pro-L-Phe-L-trp])Show SMILES O=C1N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCC[C@H]12 |r| Show InChI InChI=1S/C34H35N5O4/c40-31-27(18-22-10-3-1-4-11-22)37-33(42)30-16-9-17-39(30)34(43)29(19-23-12-5-2-6-13-23)38-32(41)28(36-31)20-24-21-35-26-15-8-7-14-25(24)26/h1-8,10-15,21,27-30,35H,9,16-20H2,(H,36,40)(H,37,42)(H,38,41)/t27-,28-,29-,30+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human cloned mu receptor by cell based assay |
Bioorg Med Chem Lett 19: 3647-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.105
BindingDB Entry DOI: 10.7270/Q2HT2Q8K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50268462

(CHEMBL506616 | c[L-Phe-D-pro-L-Phe-L-trp])Show SMILES O=C1N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCC[C@H]12 |r| Show InChI InChI=1S/C34H35N5O4/c40-31-27(18-22-10-3-1-4-11-22)37-33(42)30-16-9-17-39(30)34(43)29(19-23-12-5-2-6-13-23)38-32(41)28(36-31)20-24-21-35-26-15-8-7-14-25(24)26/h1-8,10-15,21,27-30,35H,9,16-20H2,(H,36,40)(H,37,42)(H,38,41)/t27-,28-,29-,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 451 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50268462

(CHEMBL506616 | c[L-Phe-D-pro-L-Phe-L-trp])Show SMILES O=C1N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCC[C@H]12 |r| Show InChI InChI=1S/C34H35N5O4/c40-31-27(18-22-10-3-1-4-11-22)37-33(42)30-16-9-17-39(30)34(43)29(19-23-12-5-2-6-13-23)38-32(41)28(36-31)20-24-21-35-26-15-8-7-14-25(24)26/h1-8,10-15,21,27-30,35H,9,16-20H2,(H,36,40)(H,37,42)(H,38,41)/t27-,28-,29-,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 619 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01915
BindingDB Entry DOI: 10.7270/Q22B931S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50268462

(CHEMBL506616 | c[L-Phe-D-pro-L-Phe-L-trp])Show SMILES O=C1N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCC[C@H]12 |r| Show InChI InChI=1S/C34H35N5O4/c40-31-27(18-22-10-3-1-4-11-22)37-33(42)30-16-9-17-39(30)34(43)29(19-23-12-5-2-6-13-23)38-32(41)28(36-31)20-24-21-35-26-15-8-7-14-25(24)26/h1-8,10-15,21,27-30,35H,9,16-20H2,(H,36,40)(H,37,42)(H,38,41)/t27-,28-,29-,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 619 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat cloned MOR expressed in CHO cells after 90 mins by scintillation counting analysis |
J Med Chem 56: 2178-95 (2013)
Article DOI: 10.1021/jm301783x
BindingDB Entry DOI: 10.7270/Q22808XD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50268462

(CHEMBL506616 | c[L-Phe-D-pro-L-Phe-L-trp])Show SMILES O=C1N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCC[C@H]12 |r| Show InChI InChI=1S/C34H35N5O4/c40-31-27(18-22-10-3-1-4-11-22)37-33(42)30-16-9-17-39(30)34(43)29(19-23-12-5-2-6-13-23)38-32(41)28(36-31)20-24-21-35-26-15-8-7-14-25(24)26/h1-8,10-15,21,27-30,35H,9,16-20H2,(H,36,40)(H,37,42)(H,38,41)/t27-,28-,29-,30+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at human cloned mu opioid receptor assessed as inhibition of 100 nM loperamide-stimulated GTPgammaS binding by cell based assay |
Bioorg Med Chem Lett 19: 3647-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.105
BindingDB Entry DOI: 10.7270/Q2HT2Q8K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50268462

(CHEMBL506616 | c[L-Phe-D-pro-L-Phe-L-trp])Show SMILES O=C1N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCC[C@H]12 |r| Show InChI InChI=1S/C34H35N5O4/c40-31-27(18-22-10-3-1-4-11-22)37-33(42)30-16-9-17-39(30)34(43)29(19-23-12-5-2-6-13-23)38-32(41)28(36-31)20-24-21-35-26-15-8-7-14-25(24)26/h1-8,10-15,21,27-30,35H,9,16-20H2,(H,36,40)(H,37,42)(H,38,41)/t27-,28-,29-,30+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna
Curated by ChEMBL
| Assay Description
Binding affinity to MOR (unknown origin) |
J Med Chem 59: 9255-9261 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00420
BindingDB Entry DOI: 10.7270/Q2JS9TXZ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50268462

(CHEMBL506616 | c[L-Phe-D-pro-L-Phe-L-trp])Show SMILES O=C1N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCC[C@H]12 |r| Show InChI InChI=1S/C34H35N5O4/c40-31-27(18-22-10-3-1-4-11-22)37-33(42)30-16-9-17-39(30)34(43)29(19-23-12-5-2-6-13-23)38-32(41)28(36-31)20-24-21-35-26-15-8-7-14-25(24)26/h1-8,10-15,21,27-30,35H,9,16-20H2,(H,36,40)(H,37,42)(H,38,41)/t27-,28-,29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu-opioid receptor in guinea pig brain membranes after 30 mins by scintillation counting method |
Eur J Med Chem 141: 632-647 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.012
BindingDB Entry DOI: 10.7270/Q2V98BQ2 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50268462

(CHEMBL506616 | c[L-Phe-D-pro-L-Phe-L-trp])Show SMILES O=C1N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCC[C@H]12 |r| Show InChI InChI=1S/C34H35N5O4/c40-31-27(18-22-10-3-1-4-11-22)37-33(42)30-16-9-17-39(30)34(43)29(19-23-12-5-2-6-13-23)38-32(41)28(36-31)20-24-21-35-26-15-8-7-14-25(24)26/h1-8,10-15,21,27-30,35H,9,16-20H2,(H,36,40)(H,37,42)(H,38,41)/t27-,28-,29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of DAGO from MOR in guinea pig brain membrane fraction after 30 mins by scintillation counting analysis |
J Med Chem 56: 2178-95 (2013)
Article DOI: 10.1021/jm301783x
BindingDB Entry DOI: 10.7270/Q22808XD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50268462

(CHEMBL506616 | c[L-Phe-D-pro-L-Phe-L-trp])Show SMILES O=C1N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCC[C@H]12 |r| Show InChI InChI=1S/C34H35N5O4/c40-31-27(18-22-10-3-1-4-11-22)37-33(42)30-16-9-17-39(30)34(43)29(19-23-12-5-2-6-13-23)38-32(41)28(36-31)20-24-21-35-26-15-8-7-14-25(24)26/h1-8,10-15,21,27-30,35H,9,16-20H2,(H,36,40)(H,37,42)(H,38,41)/t27-,28-,29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Adolor Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to mu opioid receptor in guinea pig brain membrane |
Bioorg Med Chem Lett 19: 3647-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.105
BindingDB Entry DOI: 10.7270/Q2HT2Q8K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data