

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50296341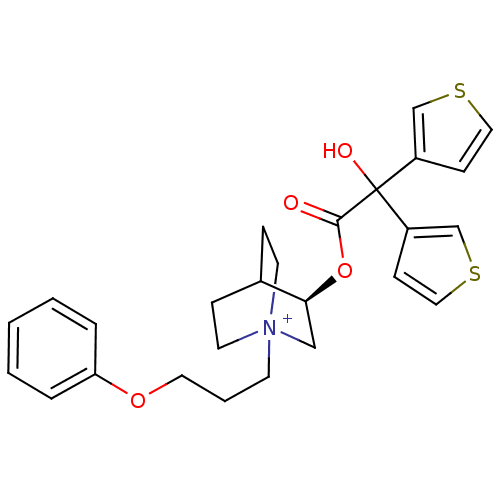 ((3R)-3-{[Hydroxy(di-3-thienyl)acetyl]oxy}-1-(3-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M3 receptor expressed in CHOK1 cells by microplate scintillation counting | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50296341 ((3R)-3-{[Hydroxy(di-3-thienyl)acetyl]oxy}-1-(3-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMIRALL, S.A. US Patent | Assay Description The binding of [3H]-NMS to human muscarinic receptors was performed according to Waelbroek et al (1990) (1). Assays were carried out at 25° C. Membra... | US Patent US9333195 (2016) BindingDB Entry DOI: 10.7270/Q2GF0SDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50296341 ((3R)-3-{[Hydroxy(di-3-thienyl)acetyl]oxy}-1-(3-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | 25 |
ALMIRALL, S.A. US Patent | Assay Description Assays were carried out at 25° C. Membrane preparations from stably transfected chinese hamster ovary-K1 cells (CHO) expressing the genes for the... | US Patent US9687478 (2017) BindingDB Entry DOI: 10.7270/Q2RV0KV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||