

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50058468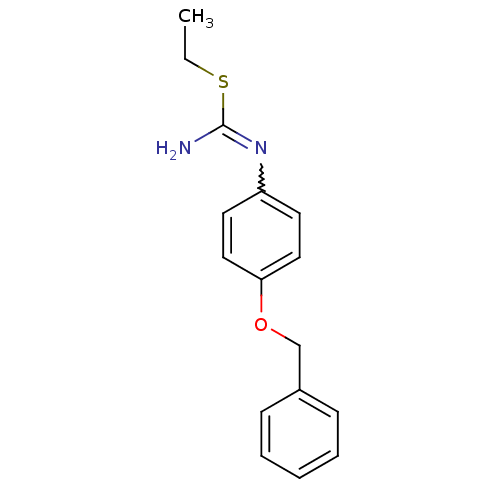 (1-(4-Benzyloxy-phenyl)-2-ethyl-isothiourea; hydrio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity against human neuronal nitric oxide synthase in brain (nNOS). | J Med Chem 40: 1901-5 (1997) Article DOI: 10.1021/jm960785c BindingDB Entry DOI: 10.7270/Q2SJ1M94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50058468 (1-(4-Benzyloxy-phenyl)-2-ethyl-isothiourea; hydrio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica Curated by ChEMBL | Assay Description Inhibition of nNOS (unknown origin) assessed as conversion of L-[3H]arginine to L-[3H]citrulline | Bioorg Med Chem 16: 6193-206 (2008) Article DOI: 10.1016/j.bmc.2008.04.036 BindingDB Entry DOI: 10.7270/Q2611040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||