

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50147745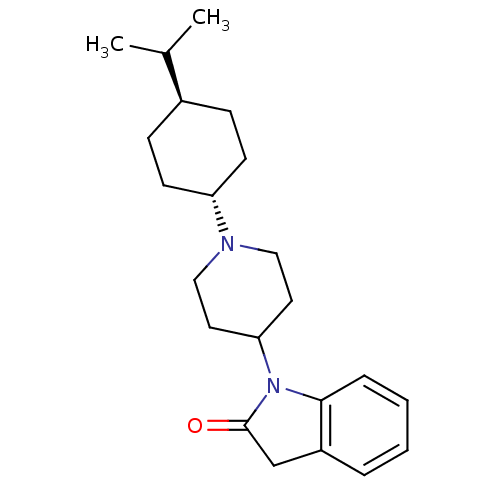 (1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-1,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astraea Therapeutics, LLC. Curated by ChEMBL | Assay Description Displacement of [3H] N/OFQ from human nociceptin receptor transfected in CHO cells | Bioorg Med Chem Lett 23: 3308-13 (2013) Article DOI: 10.1016/j.bmcl.2013.03.101 BindingDB Entry DOI: 10.7270/Q2T43VHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50147745 (1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-1,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity against human Nociceptin receptor on CHO cell membranes by [3H]N/OFQ displacement. | J Med Chem 47: 2973-6 (2004) Article DOI: 10.1021/jm034249d BindingDB Entry DOI: 10.7270/Q2KW5FHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50147745 (1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-1,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
Astraea Therapeutics, LLC. Curated by ChEMBL | Assay Description Agonist activity at human nociceptin receptor transfected in CHO cells assessed as stimulation of [35S]GTPgammaS binding by liquid scintillation coun... | Bioorg Med Chem Lett 23: 3308-13 (2013) Article DOI: 10.1016/j.bmcl.2013.03.101 BindingDB Entry DOI: 10.7270/Q2T43VHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50147745 (1-[1-(4-Isopropyl-cyclohexyl)-piperidin-4-yl]-1,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 117 | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Concentration required for stimulation of [35S]-GTP-gammaS, binding to human Nociceptin receptor in cell membranes | J Med Chem 47: 2973-6 (2004) Article DOI: 10.1021/jm034249d BindingDB Entry DOI: 10.7270/Q2KW5FHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||