

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Orexin receptor type 2 (Rattus norvegicus (Rat)) | BDBM50417257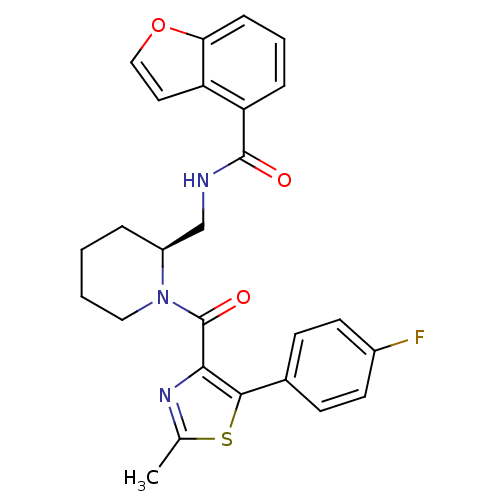 (SB-649868) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0776 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre Curated by ChEMBL | Assay Description Antagonist activity at recombinant rat OX2R expressed in CHO cells by FLIPR calcium based functional assay | Bioorg Med Chem Lett 21: 5562-7 (2011) Article DOI: 10.1016/j.bmcl.2011.06.086 BindingDB Entry DOI: 10.7270/Q29K4CGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50417257 (SB-649868) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre Curated by ChEMBL | Assay Description Antagonist activity at recombinant human OX2R expressed in CHO cells by FLIPR calcium based functional assay | Bioorg Med Chem Lett 21: 5562-7 (2011) Article DOI: 10.1016/j.bmcl.2011.06.086 BindingDB Entry DOI: 10.7270/Q29K4CGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50417257 (SB-649868) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity against human orexin 2 receptor expressed in CHO cells assessed as effect in calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 20: 6405-7 (2010) Article DOI: 10.1016/j.bmcl.2010.09.090 BindingDB Entry DOI: 10.7270/Q2W37WKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50417257 (SB-649868) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research& Development Curated by ChEMBL | Assay Description Binding affinity to orexin receptor 2 (unknown origin) | Bioorg Med Chem Lett 23: 4761-9 (2013) Article DOI: 10.1016/j.bmcl.2013.06.057 BindingDB Entry DOI: 10.7270/Q25140NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50417257 (SB-649868) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 1.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]almorexant from recombinant human OX2R expressed in CHO cells | Bioorg Med Chem Lett 21: 5562-7 (2011) Article DOI: 10.1016/j.bmcl.2011.06.086 BindingDB Entry DOI: 10.7270/Q29K4CGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50417257 (SB-649868) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd Curated by ChEMBL | Assay Description Displacement of [125I]-Orexin A from human OX2R expressed in CHO cells after 30 mins by topcount analysis | Bioorg Med Chem 22: 6071-88 (2014) Article DOI: 10.1016/j.bmc.2014.08.034 BindingDB Entry DOI: 10.7270/Q20R9R0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||