

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062286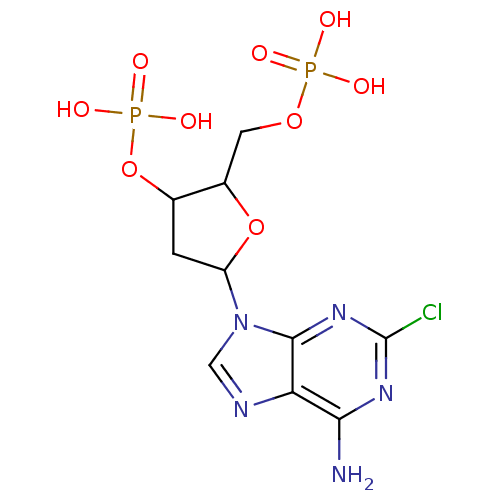 (CHEMBL44408 | Phosphoric acid mono-[5-(6-amino-2-c...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATP | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062286 (CHEMBL44408 | Phosphoric acid mono-[5-(6-amino-2-c...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description In vitro antagonist activity at P2Y1 receptor in turkey erythrocyte membranes. | J Med Chem 42: 1625-38 (1999) Article DOI: 10.1021/jm980657j BindingDB Entry DOI: 10.7270/Q28C9WZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062286 (CHEMBL44408 | Phosphoric acid mono-[5-(6-amino-2-c...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 651 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Concentration at which 50% of the maximal effect (stimulation of PLC at P2Y1 receptor in the turkey erythrocyte membranes) is reached | J Med Chem 42: 1625-38 (1999) Article DOI: 10.1021/jm980657j BindingDB Entry DOI: 10.7270/Q28C9WZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062286 (CHEMBL44408 | Phosphoric acid mono-[5-(6-amino-2-c...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 651 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Agonist activity at P2Y1 receptor measured as capacity to stimulate 50% phospholipase C in turkey erythrocyte membranes | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||