 Found 4 hits Enz. Inhib. hit(s) with Target = 'Peptidyl-prolyl cis-trans isomerase FKBP1A' and Ligand = 'BDBM50067004'
Found 4 hits Enz. Inhib. hit(s) with Target = 'Peptidyl-prolyl cis-trans isomerase FKBP1A' and Ligand = 'BDBM50067004' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50067004
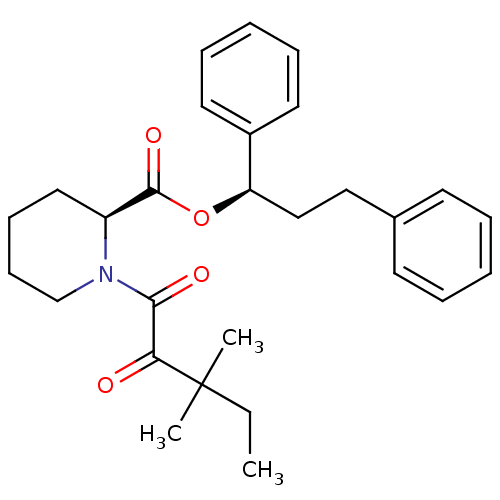
((S)-1-(3,3-Dimethyl-2-oxo-pentanoyl)-piperidine-2-...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H35NO4/c1-4-28(2,3)25(30)26(31)29-20-12-11-17-23(29)27(32)33-24(22-15-9-6-10-16-22)19-18-21-13-7-5-8-14-21/h5-10,13-16,23-24H,4,11-12,17-20H2,1-3H3/t23-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wu£rzburg
Curated by ChEMBL
| Assay Description
Inhibition of FKBP12 |
J Med Chem 54: 277-83 (2011)
Article DOI: 10.1021/jm101156y
BindingDB Entry DOI: 10.7270/Q2GF0VG2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50067004

((S)-1-(3,3-Dimethyl-2-oxo-pentanoyl)-piperidine-2-...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H35NO4/c1-4-28(2,3)25(30)26(31)29-20-12-11-17-23(29)27(32)33-24(22-15-9-6-10-16-22)19-18-21-13-7-5-8-14-21/h5-10,13-16,23-24H,4,11-12,17-20H2,1-3H3/t23-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human FKBP12 expressed in Escherichia coli using succinyl-Ala-Phe-Pro-Phe-4-nitroanilide as substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00911
BindingDB Entry DOI: 10.7270/Q22V2KW9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50067004

((S)-1-(3,3-Dimethyl-2-oxo-pentanoyl)-piperidine-2-...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H35NO4/c1-4-28(2,3)25(30)26(31)29-20-12-11-17-23(29)27(32)33-24(22-15-9-6-10-16-22)19-18-21-13-7-5-8-14-21/h5-10,13-16,23-24H,4,11-12,17-20H2,1-3H3/t23-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
The compound was tested for binding affinity against rotamase |
J Med Chem 41: 3928-39 (1998)
Article DOI: 10.1021/jm980062o
BindingDB Entry DOI: 10.7270/Q2SB44W1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50067004

((S)-1-(3,3-Dimethyl-2-oxo-pentanoyl)-piperidine-2-...)Show SMILES CCC(C)(C)C(=O)C(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H35NO4/c1-4-28(2,3)25(30)26(31)29-20-12-11-17-23(29)27(32)33-24(22-15-9-6-10-16-22)19-18-21-13-7-5-8-14-21/h5-10,13-16,23-24H,4,11-12,17-20H2,1-3H3/t23-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
The compound was tested for binding affinity against rotamase |
J Med Chem 41: 3928-39 (1998)
Article DOI: 10.1021/jm980062o
BindingDB Entry DOI: 10.7270/Q2SB44W1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data