

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasminogen (Homo sapiens (Human)) | BDBM23891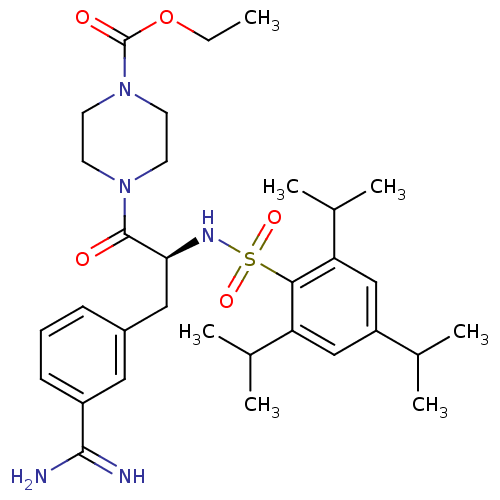 (3-amidinophenylalanine deriv., 35 | CHEMBL107955 |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Chemistry GmbH | Assay Description The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af... | J Med Chem 49: 4116-26 (2006) Article DOI: 10.1021/jm051272l BindingDB Entry DOI: 10.7270/Q21C1V64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM23891 (3-amidinophenylalanine deriv., 35 | CHEMBL107955 |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Jena Curated by ChEMBL | Assay Description Compound was tested for inhibition of plasmin | Bioorg Med Chem Lett 9: 3147-52 (1999) BindingDB Entry DOI: 10.7270/Q2RB73S3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM23891 (3-amidinophenylalanine deriv., 35 | CHEMBL107955 |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human plasma plasmin using pyroGlu-Pro-Arg-p-NA.HCl as substrate preincubated for 15 mins prior sustrate addition measured for 10 mins ... | Bioorg Med Chem 20: 1557-68 (2012) Article DOI: 10.1016/j.bmc.2011.12.040 BindingDB Entry DOI: 10.7270/Q2QR4XK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM23891 (3-amidinophenylalanine deriv., 35 | CHEMBL107955 |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of human plasmin | J Med Chem 63: 1445-1472 (2020) Article DOI: 10.1021/acs.jmedchem.9b01060 BindingDB Entry DOI: 10.7270/Q2VQ361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM23891 (3-amidinophenylalanine deriv., 35 | CHEMBL107955 |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of plasmin (unknown origin) | J Med Chem 62: 2172-2183 (2019) Article DOI: 10.1021/acs.jmedchem.8b01908 BindingDB Entry DOI: 10.7270/Q24F1V6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||