

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50168761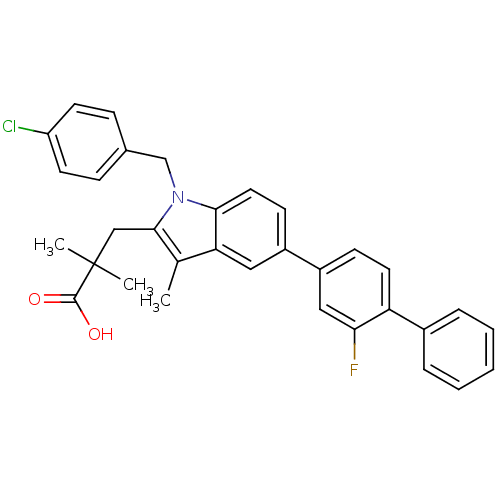 (3-(1-(4-chlorobenzyl)-5-(2-fluorobiphenyl-4-yl)-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant mPGES-1 using PGH2 as substrate incubated 20 mins prior to substrate addition measured after 30 secs by EIA | Bioorg Med Chem Lett 23: 907-11 (2013) Article DOI: 10.1016/j.bmcl.2012.10.040 BindingDB Entry DOI: 10.7270/Q2Z3210H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50168761 (3-(1-(4-chlorobenzyl)-5-(2-fluorobiphenyl-4-yl)-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 in cell-free system assessed as inhibition of conversion of PGH2 to PGE2 by HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50168761 (3-(1-(4-chlorobenzyl)-5-(2-fluorobiphenyl-4-yl)-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL-1beta treated human A549 cell microsomal membrane assessed as residual enzyme activity after 1 min by measuring PGE2 level... | J Med Chem 52: 4968-72 (2009) Article DOI: 10.1021/jm900481c BindingDB Entry DOI: 10.7270/Q29C6XGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50168761 (3-(1-(4-chlorobenzyl)-5-(2-fluorobiphenyl-4-yl)-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||