

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase Src (Gallus gallus (Chicken)) | BDBM3101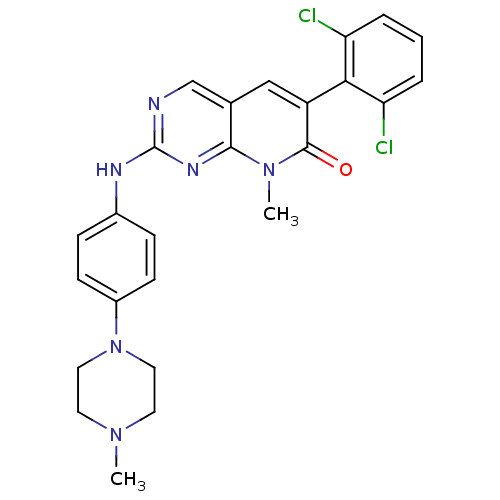 (2-aminopyrido[2,3-d]pyrimidin-7(8H)-one 68 | 6-(2,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of chicken c-Src tyrosine kinase | J Med Chem 41: 1752-63 (1998) Article DOI: 10.1021/jm970634p BindingDB Entry DOI: 10.7270/Q26T0KQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM3101 (2-aminopyrido[2,3-d]pyrimidin-7(8H)-one 68 | 6-(2,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 3276-92 (1998) Article DOI: 10.1021/jm9802259 BindingDB Entry DOI: 10.7270/Q27P8WK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||