

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293079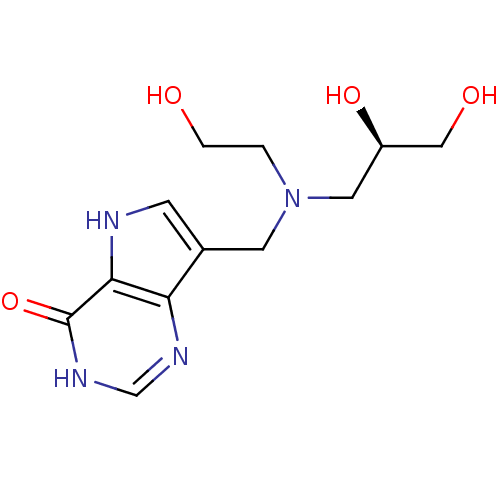 (7-({[(2S)-2,3-Dihydroxypropyl](2-hydroxyethyl)amin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human PNP by xanthine-oxidase coupled assay | J Med Chem 52: 1126-43 (2009) Article DOI: 10.1021/jm801421q BindingDB Entry DOI: 10.7270/Q2QR4Z18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293079 (7-({[(2S)-2,3-Dihydroxypropyl](2-hydroxyethyl)amin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 165 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50293079 (7-({[(2S)-2,3-Dihydroxypropyl](2-hydroxyethyl)amin...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 550 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||