

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50099963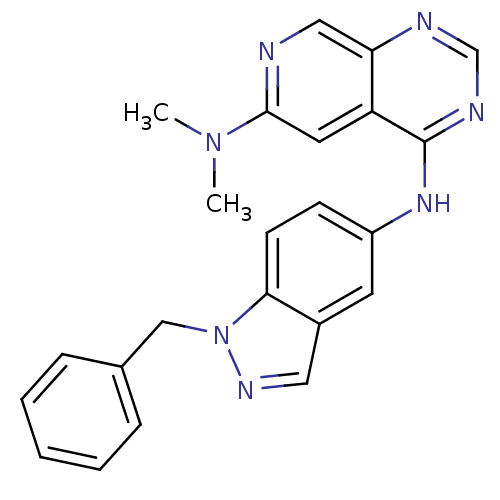 (CHEMBL30432 | N*4*-(1-Benzyl-1H-indazol-5-yl)-N*6*...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Inhibition of ErbB-2 intracellular domain (unknown origin) purified from a baculovirus expression system using Biotin-(amino hexonoic acid)-EEEEYFELV... | Eur J Med Chem 95: 76-95 (2015) Article DOI: 10.1016/j.ejmech.2015.03.029 BindingDB Entry DOI: 10.7270/Q2PR7XP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50099963 (CHEMBL30432 | N*4*-(1-Benzyl-1H-indazol-5-yl)-N*6*...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of erbB2 overexpressing BT 474 cell proliferation | Bioorg Med Chem Lett 11: 1401-5 (2001) BindingDB Entry DOI: 10.7270/Q2W37VKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50099963 (CHEMBL30432 | N*4*-(1-Benzyl-1H-indazol-5-yl)-N*6*...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of erbB2 overexpressing HB4a.e5.2 cell proliferation | Bioorg Med Chem Lett 11: 1401-5 (2001) BindingDB Entry DOI: 10.7270/Q2W37VKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Rattus norvegicus) | BDBM50099963 (CHEMBL30432 | N*4*-(1-Benzyl-1H-indazol-5-yl)-N*6*...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of erbB2 overexpressing Calu3 cell proliferation | Bioorg Med Chem Lett 11: 1401-5 (2001) BindingDB Entry DOI: 10.7270/Q2W37VKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Rattus norvegicus) | BDBM50099963 (CHEMBL30432 | N*4*-(1-Benzyl-1H-indazol-5-yl)-N*6*...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biomet. Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Inhibition of erbB2 overexpressing Calu3 cell proliferation | Bioorg Med Chem Lett 11: 1401-5 (2001) BindingDB Entry DOI: 10.7270/Q2W37VKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||