 Found 7 hits Enz. Inhib. hit(s) with Target = 'Ribosomal protein S6 kinase alpha-3' and Ligand = 'BDBM50248765'
Found 7 hits Enz. Inhib. hit(s) with Target = 'Ribosomal protein S6 kinase alpha-3' and Ligand = 'BDBM50248765' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50248765
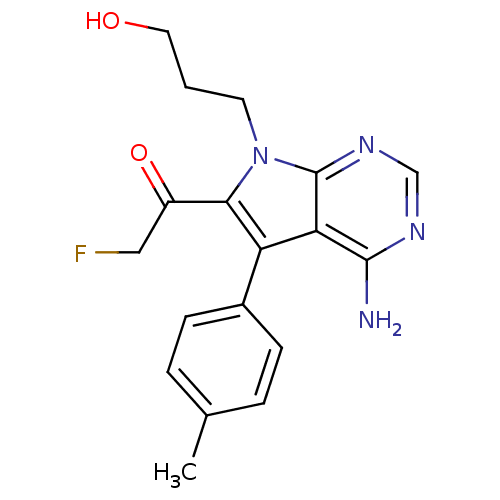
(1-(4-amino-7-(3-hydroxypropyl)-5-p-tolyl-7H-pyrrol...)Show SMILES Cc1ccc(cc1)-c1c(C(=O)CF)n(CCCO)c2ncnc(N)c12 Show InChI InChI=1S/C18H19FN4O2/c1-11-3-5-12(6-4-11)14-15-17(20)21-10-22-18(15)23(7-2-8-24)16(14)13(25)9-19/h3-6,10,24H,2,7-9H2,1H3,(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 after 60 mins |
J Med Chem 54: 3564-74 (2011)
Article DOI: 10.1021/jm200139j
BindingDB Entry DOI: 10.7270/Q26M374H |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50248765

(1-(4-amino-7-(3-hydroxypropyl)-5-p-tolyl-7H-pyrrol...)Show SMILES Cc1ccc(cc1)-c1c(C(=O)CF)n(CCCO)c2ncnc(N)c12 Show InChI InChI=1S/C18H19FN4O2/c1-11-3-5-12(6-4-11)14-15-17(20)21-10-22-18(15)23(7-2-8-24)16(14)13(25)9-19/h3-6,10,24H,2,7-9H2,1H3,(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Covalution Pharma BV
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 transfected in HEK293 cells |
J Med Chem 55: 6243-62 (2012)
Article DOI: 10.1021/jm3003203
BindingDB Entry DOI: 10.7270/Q2X92CGP |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50248765

(1-(4-amino-7-(3-hydroxypropyl)-5-p-tolyl-7H-pyrrol...)Show SMILES Cc1ccc(cc1)-c1c(C(=O)CF)n(CCCO)c2ncnc(N)c12 Show InChI InChI=1S/C18H19FN4O2/c1-11-3-5-12(6-4-11)14-15-17(20)21-10-22-18(15)23(7-2-8-24)16(14)13(25)9-19/h3-6,10,24H,2,7-9H2,1H3,(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of His6-tagged RSK2 C-terminal domian expressed in Escherichia coli after 30 mins |
Nat Chem Biol 3: 156-60 (2007)
Article DOI: 10.1038/nchembio859
BindingDB Entry DOI: 10.7270/Q22B8Z60 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50248765

(1-(4-amino-7-(3-hydroxypropyl)-5-p-tolyl-7H-pyrrol...)Show SMILES Cc1ccc(cc1)-c1c(C(=O)CF)n(CCCO)c2ncnc(N)c12 Show InChI InChI=1S/C18H19FN4O2/c1-11-3-5-12(6-4-11)14-15-17(20)21-10-22-18(15)23(7-2-8-24)16(14)13(25)9-19/h3-6,10,24H,2,7-9H2,1H3,(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of RSK2 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00969
BindingDB Entry DOI: 10.7270/Q2WD44DC |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50248765

(1-(4-amino-7-(3-hydroxypropyl)-5-p-tolyl-7H-pyrrol...)Show SMILES Cc1ccc(cc1)-c1c(C(=O)CF)n(CCCO)c2ncnc(N)c12 Show InChI InChI=1S/C18H19FN4O2/c1-11-3-5-12(6-4-11)14-15-17(20)21-10-22-18(15)23(7-2-8-24)16(14)13(25)9-19/h3-6,10,24H,2,7-9H2,1H3,(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of His6x-tagged RSK2 C-terminal domain (unknown origin) expressed in Escherichia coli |
Bioorg Med Chem 21: 1724-34 (2013)
Article DOI: 10.1016/j.bmc.2013.01.047
BindingDB Entry DOI: 10.7270/Q2GQ704N |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50248765

(1-(4-amino-7-(3-hydroxypropyl)-5-p-tolyl-7H-pyrrol...)Show SMILES Cc1ccc(cc1)-c1c(C(=O)CF)n(CCCO)c2ncnc(N)c12 Show InChI InChI=1S/C18H19FN4O2/c1-11-3-5-12(6-4-11)14-15-17(20)21-10-22-18(15)23(7-2-8-24)16(14)13(25)9-19/h3-6,10,24H,2,7-9H2,1H3,(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 C-terminal kinase domain (unknown origin) expressed in Escherichia coli preincubated for 30 mins |
Science 308: 1318-21 (2005)
Article DOI: 10.1126/science1108367
BindingDB Entry DOI: 10.7270/Q20G3JX9 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50248765

(1-(4-amino-7-(3-hydroxypropyl)-5-p-tolyl-7H-pyrrol...)Show SMILES Cc1ccc(cc1)-c1c(C(=O)CF)n(CCCO)c2ncnc(N)c12 Show InChI InChI=1S/C18H19FN4O2/c1-11-3-5-12(6-4-11)14-15-17(20)21-10-22-18(15)23(7-2-8-24)16(14)13(25)9-19/h3-6,10,24H,2,7-9H2,1H3,(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of PMA-stimulated RSK2 phosphorylation in HEK293 cells after 1 hr by western blotting analysis |
Nat Chem Biol 3: 156-60 (2007)
Article DOI: 10.1038/nchembio859
BindingDB Entry DOI: 10.7270/Q22B8Z60 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data