

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM197714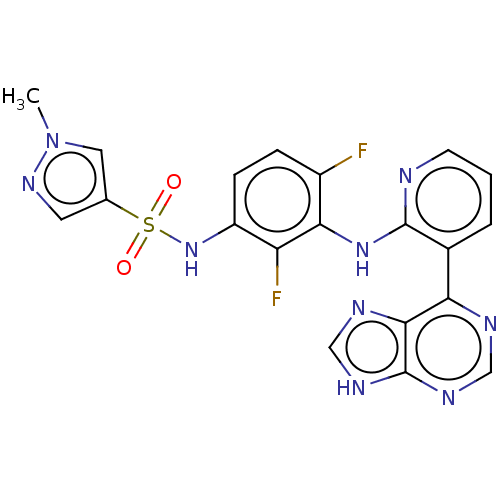 (US9216981, 43) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay | Bioorg Med Chem 24: 2215-34 (2016) Article DOI: 10.1016/j.bmc.2016.03.055 BindingDB Entry DOI: 10.7270/Q21C1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM197714 (US9216981, 43) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of wild type B-Raf (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay | Bioorg Med Chem 24: 2215-34 (2016) Article DOI: 10.1016/j.bmc.2016.03.055 BindingDB Entry DOI: 10.7270/Q21C1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM197714 (US9216981, 43) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.2 | 30 |
Medpacto, Inc. US Patent | Assay Description To check the B-Raf kinase inhibitory activity of the compounds of the present invention, the following experiments were conducted.(1) Serial Signalin... | US Patent US9216981 (2015) BindingDB Entry DOI: 10.7270/Q2BC3XCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM197714 (US9216981, 43) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of B-Raf V600E mutant in human A375 cells assessed as ERK phosphorylation preincubated for 1 hr by Western blot method | Bioorg Med Chem 24: 2215-34 (2016) Article DOI: 10.1016/j.bmc.2016.03.055 BindingDB Entry DOI: 10.7270/Q21C1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM197714 (US9216981, 43) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.22E+3 | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of wild type B-Raf in human MIAPaCa2 cells assessed as reduction in ERK phosphorylation preincubated for 1 hr by Western blot method | Bioorg Med Chem 24: 2215-34 (2016) Article DOI: 10.1016/j.bmc.2016.03.055 BindingDB Entry DOI: 10.7270/Q21C1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM197714 (US9216981, 43) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.37E+3 | n/a | n/a | n/a | 37 |
Medpacto, Inc. US Patent | Assay Description To check the B-Raf cell activity inhibitory capability of the compounds of the present invention, the following experiments were conducted in A375P... | US Patent US9216981 (2015) BindingDB Entry DOI: 10.7270/Q2BC3XCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||