 Found 9 hits Enz. Inhib. hit(s) with Target = 'Somatostatin receptor type 3' and Ligand = 'BDBM82463'
Found 9 hits Enz. Inhib. hit(s) with Target = 'Somatostatin receptor type 3' and Ligand = 'BDBM82463' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM82463
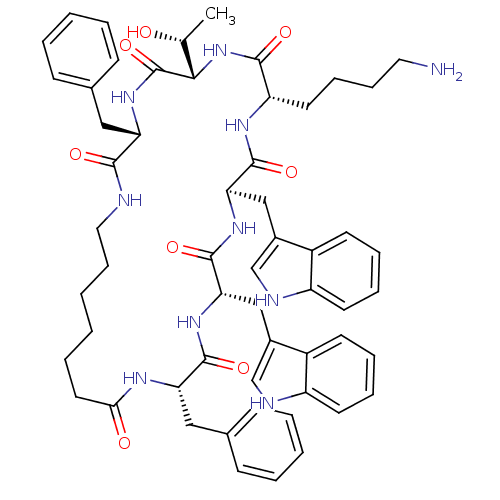
(Cyclo[L-Trp-D-Trp-L-Lys-L-Thr-L-Phe-(7-amino*hepta...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)CCCCCCNC(=O)[C@H](Cc2ccccc2)NC1=O Show InChI InChI=1S/C57H70N10O8/c1-36(68)51-57(75)66-46(30-37-18-6-4-7-19-37)52(70)59-29-17-3-2-10-27-50(69)62-47(31-38-20-8-5-9-21-38)54(72)64-49(33-40-35-61-44-25-14-12-23-42(40)44)56(74)65-48(32-39-34-60-43-24-13-11-22-41(39)43)55(73)63-45(53(71)67-51)26-15-16-28-58/h4-9,11-14,18-25,34-36,45-49,51,60-61,68H,2-3,10,15-17,26-33,58H2,1H3,(H,59,70)(H,62,69)(H,63,73)(H,64,72)(H,65,74)(H,66,75)(H,67,71)/t36-,45+,46+,47+,48-,49+,51+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 360: 488-99 (1999)
Article DOI: 10.1007/s002109900141
BindingDB Entry DOI: 10.7270/Q2Q23XTW |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM82463

(Cyclo[L-Trp-D-Trp-L-Lys-L-Thr-L-Phe-(7-amino*hepta...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)CCCCCCNC(=O)[C@H](Cc2ccccc2)NC1=O Show InChI InChI=1S/C57H70N10O8/c1-36(68)51-57(75)66-46(30-37-18-6-4-7-19-37)52(70)59-29-17-3-2-10-27-50(69)62-47(31-38-20-8-5-9-21-38)54(72)64-49(33-40-35-61-44-25-14-12-23-42(40)44)56(74)65-48(32-39-34-60-43-24-13-11-22-41(39)43)55(73)63-45(53(71)67-51)26-15-16-28-58/h4-9,11-14,18-25,34-36,45-49,51,60-61,68H,2-3,10,15-17,26-33,58H2,1H3,(H,59,70)(H,62,69)(H,63,73)(H,64,72)(H,65,74)(H,66,75)(H,67,71)/t36-,45+,46+,47+,48-,49+,51+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 357: 483-9 (1998)
Article DOI: 10.1007/pl00005197
BindingDB Entry DOI: 10.7270/Q25H7DTQ |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM82463

(Cyclo[L-Trp-D-Trp-L-Lys-L-Thr-L-Phe-(7-amino*hepta...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)CCCCCCNC(=O)[C@H](Cc2ccccc2)NC1=O Show InChI InChI=1S/C57H70N10O8/c1-36(68)51-57(75)66-46(30-37-18-6-4-7-19-37)52(70)59-29-17-3-2-10-27-50(69)62-47(31-38-20-8-5-9-21-38)54(72)64-49(33-40-35-61-44-25-14-12-23-42(40)44)56(74)65-48(32-39-34-60-43-24-13-11-22-41(39)43)55(73)63-45(53(71)67-51)26-15-16-28-58/h4-9,11-14,18-25,34-36,45-49,51,60-61,68H,2-3,10,15-17,26-33,58H2,1H3,(H,59,70)(H,62,69)(H,63,73)(H,64,72)(H,65,74)(H,66,75)(H,67,71)/t36-,45+,46+,47+,48-,49+,51+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 360: 488-99 (1999)
Article DOI: 10.1007/s002109900141
BindingDB Entry DOI: 10.7270/Q2Q23XTW |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM82463

(Cyclo[L-Trp-D-Trp-L-Lys-L-Thr-L-Phe-(7-amino*hepta...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)CCCCCCNC(=O)[C@H](Cc2ccccc2)NC1=O Show InChI InChI=1S/C57H70N10O8/c1-36(68)51-57(75)66-46(30-37-18-6-4-7-19-37)52(70)59-29-17-3-2-10-27-50(69)62-47(31-38-20-8-5-9-21-38)54(72)64-49(33-40-35-61-44-25-14-12-23-42(40)44)56(74)65-48(32-39-34-60-43-24-13-11-22-41(39)43)55(73)63-45(53(71)67-51)26-15-16-28-58/h4-9,11-14,18-25,34-36,45-49,51,60-61,68H,2-3,10,15-17,26-33,58H2,1H3,(H,59,70)(H,62,69)(H,63,73)(H,64,72)(H,65,74)(H,66,75)(H,67,71)/t36-,45+,46+,47+,48-,49+,51+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd
Curated by PDSP Ki Database
| |
Metab Clin Exp 45: 17-20 (1996)
Article DOI: 10.1016/s0026-0495(96)90072-4
BindingDB Entry DOI: 10.7270/Q2WQ02B7 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM82463

(Cyclo[L-Trp-D-Trp-L-Lys-L-Thr-L-Phe-(7-amino*hepta...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)CCCCCCNC(=O)[C@H](Cc2ccccc2)NC1=O Show InChI InChI=1S/C57H70N10O8/c1-36(68)51-57(75)66-46(30-37-18-6-4-7-19-37)52(70)59-29-17-3-2-10-27-50(69)62-47(31-38-20-8-5-9-21-38)54(72)64-49(33-40-35-61-44-25-14-12-23-42(40)44)56(74)65-48(32-39-34-60-43-24-13-11-22-41(39)43)55(73)63-45(53(71)67-51)26-15-16-28-58/h4-9,11-14,18-25,34-36,45-49,51,60-61,68H,2-3,10,15-17,26-33,58H2,1H3,(H,59,70)(H,62,69)(H,63,73)(H,64,72)(H,65,74)(H,66,75)(H,67,71)/t36-,45+,46+,47+,48-,49+,51+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24.0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 360: 488-99 (1999)
Article DOI: 10.1007/s002109900141
BindingDB Entry DOI: 10.7270/Q2Q23XTW |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM82463

(Cyclo[L-Trp-D-Trp-L-Lys-L-Thr-L-Phe-(7-amino*hepta...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)CCCCCCNC(=O)[C@H](Cc2ccccc2)NC1=O Show InChI InChI=1S/C57H70N10O8/c1-36(68)51-57(75)66-46(30-37-18-6-4-7-19-37)52(70)59-29-17-3-2-10-27-50(69)62-47(31-38-20-8-5-9-21-38)54(72)64-49(33-40-35-61-44-25-14-12-23-42(40)44)56(74)65-48(32-39-34-60-43-24-13-11-22-41(39)43)55(73)63-45(53(71)67-51)26-15-16-28-58/h4-9,11-14,18-25,34-36,45-49,51,60-61,68H,2-3,10,15-17,26-33,58H2,1H3,(H,59,70)(H,62,69)(H,63,73)(H,64,72)(H,65,74)(H,66,75)(H,67,71)/t36-,45+,46+,47+,48-,49+,51+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24.0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 357: 483-9 (1998)
Article DOI: 10.1007/pl00005197
BindingDB Entry DOI: 10.7270/Q25H7DTQ |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(MOUSE) | BDBM82463

(Cyclo[L-Trp-D-Trp-L-Lys-L-Thr-L-Phe-(7-amino*hepta...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)CCCCCCNC(=O)[C@H](Cc2ccccc2)NC1=O Show InChI InChI=1S/C57H70N10O8/c1-36(68)51-57(75)66-46(30-37-18-6-4-7-19-37)52(70)59-29-17-3-2-10-27-50(69)62-47(31-38-20-8-5-9-21-38)54(72)64-49(33-40-35-61-44-25-14-12-23-42(40)44)56(74)65-48(32-39-34-60-43-24-13-11-22-41(39)43)55(73)63-45(53(71)67-51)26-15-16-28-58/h4-9,11-14,18-25,34-36,45-49,51,60-61,68H,2-3,10,15-17,26-33,58H2,1H3,(H,59,70)(H,62,69)(H,63,73)(H,64,72)(H,65,74)(H,66,75)(H,67,71)/t36-,45+,46+,47+,48-,49+,51+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 43: 838-44 (1993)
BindingDB Entry DOI: 10.7270/Q21N7ZN7 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(MOUSE) | BDBM82463

(Cyclo[L-Trp-D-Trp-L-Lys-L-Thr-L-Phe-(7-amino*hepta...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)CCCCCCNC(=O)[C@H](Cc2ccccc2)NC1=O Show InChI InChI=1S/C57H70N10O8/c1-36(68)51-57(75)66-46(30-37-18-6-4-7-19-37)52(70)59-29-17-3-2-10-27-50(69)62-47(31-38-20-8-5-9-21-38)54(72)64-49(33-40-35-61-44-25-14-12-23-42(40)44)56(74)65-48(32-39-34-60-43-24-13-11-22-41(39)43)55(73)63-45(53(71)67-51)26-15-16-28-58/h4-9,11-14,18-25,34-36,45-49,51,60-61,68H,2-3,10,15-17,26-33,58H2,1H3,(H,59,70)(H,62,69)(H,63,73)(H,64,72)(H,65,74)(H,66,75)(H,67,71)/t36-,45+,46+,47+,48-,49+,51+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd.
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 350: 441-53 (1994)
Article DOI: 10.1007/BF00173012
BindingDB Entry DOI: 10.7270/Q2XD105R |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(RAT) | BDBM82463

(Cyclo[L-Trp-D-Trp-L-Lys-L-Thr-L-Phe-(7-amino*hepta...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)CCCCCCNC(=O)[C@H](Cc2ccccc2)NC1=O Show InChI InChI=1S/C57H70N10O8/c1-36(68)51-57(75)66-46(30-37-18-6-4-7-19-37)52(70)59-29-17-3-2-10-27-50(69)62-47(31-38-20-8-5-9-21-38)54(72)64-49(33-40-35-61-44-25-14-12-23-42(40)44)56(74)65-48(32-39-34-60-43-24-13-11-22-41(39)43)55(73)63-45(53(71)67-51)26-15-16-28-58/h4-9,11-14,18-25,34-36,45-49,51,60-61,68H,2-3,10,15-17,26-33,58H2,1H3,(H,59,70)(H,62,69)(H,63,73)(H,64,72)(H,65,74)(H,66,75)(H,67,71)/t36-,45+,46+,47+,48-,49+,51+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
German Institute of Human Nutrition
Curated by PDSP Ki Database
| |
Rev Physiol Biochem Pharmacol 133: 55-108 (1998)
Article DOI: 10.1007/bfb0000613
BindingDB Entry DOI: 10.7270/Q22R3Q6B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data