

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50066659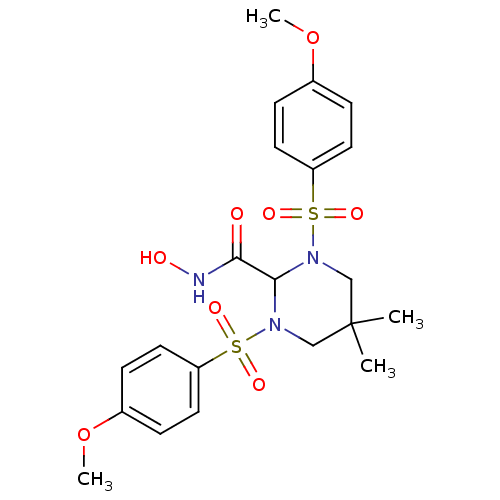 (1,3-BIS-(4-METHOXY-BENZENESULFONYL)-5,5-DIMETHYL-H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description Inhibition of matrix metalloprotease-3 (MMP-3). | J Med Chem 43: 305-41 (2000) Checked by Author BindingDB Entry DOI: 10.7270/Q2JD4XH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50066659 (1,3-BIS-(4-METHOXY-BENZENESULFONYL)-5,5-DIMETHYL-H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States. Curated by ChEMBL | Assay Description Inhibition of truncated human recombinant MMP3 catalytic domain expressed in Escherichia coli BL21(DE3) after 3 hrs in presence of [H]-transferrin | J Med Chem 61: 2166-2210 (2018) Article DOI: 10.1021/acs.jmedchem.7b00315 BindingDB Entry DOI: 10.7270/Q2B56N68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50066659 (1,3-BIS-(4-METHOXY-BENZENESULFONYL)-5,5-DIMETHYL-H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Matrix metalloprotease-3 | J Med Chem 41: 3568-71 (1998) Article DOI: 10.1021/jm980253r BindingDB Entry DOI: 10.7270/Q22F7P4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50066659 (1,3-BIS-(4-METHOXY-BENZENESULFONYL)-5,5-DIMETHYL-H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB Article PubMed | n/a | n/a | 28.6 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant MMP3 catalytic domain incubated for 20 mins by fluorimetric assay | Bioorg Med Chem 20: 4164-71 (2012) Article DOI: 10.1016/j.bmc.2012.04.063 BindingDB Entry DOI: 10.7270/Q22808NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||