

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stromelysin-1 (Homo sapiens (Human)) | BDBM92445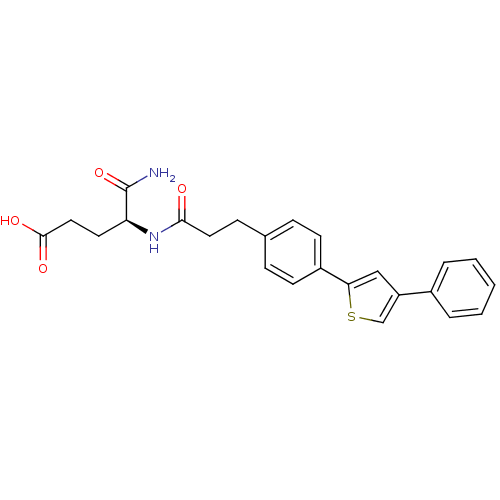 (Inhibitor, 10 | US8691753, 105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM92445 (Inhibitor, 10 | US8691753, 105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | -31.5 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
Commissariat á l'Energie Atomique | Assay Description Enzyme assay using human matrix metalloproteases or ADAMTS. | J Biol Chem 287: 26647-56 (2012) Article DOI: 10.1074/jbc.M112.380782 BindingDB Entry DOI: 10.7270/Q2H993SB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||