

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Telomerase reverse transcriptase (Homo sapiens (Human)) | BDBM50080840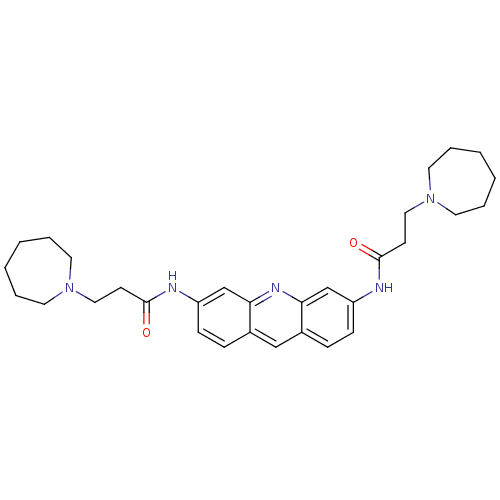 (3,6-Bis[3-(azepan-1-yl)propionamido]acridine | 3,6...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Ability to inhibit human telomerase in a modified cell-free TRAP assay using extracts from A2780 cell line | Bioorg Med Chem Lett 9: 2463-8 (1999) BindingDB Entry DOI: 10.7270/Q2H41QN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Telomerase reverse transcriptase (Homo sapiens (Human)) | BDBM50080840 (3,6-Bis[3-(azepan-1-yl)propionamido]acridine | 3,6...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central University of Las Villas Curated by ChEMBL | Assay Description Inhibition of telomerase in human A2780 cells by TRAP assay | Eur J Med Chem 44: 4826-40 (2009) Article DOI: 10.1016/j.ejmech.2009.07.029 BindingDB Entry DOI: 10.7270/Q20R9PHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Telomerase reverse transcriptase (Homo sapiens (Human)) | BDBM50080840 (3,6-Bis[3-(azepan-1-yl)propionamido]acridine | 3,6...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibitory activity against Telomerase evaluated by TRAP Assay studies. | J Med Chem 42: 4538-46 (1999) BindingDB Entry DOI: 10.7270/Q2J103VC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Telomerase reverse transcriptase (Homo sapiens (Human)) | BDBM50080840 (3,6-Bis[3-(azepan-1-yl)propionamido]acridine | 3,6...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article | n/a | n/a | 3.09E+9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of telomerase in Homo sapiens (human) A549 cells after 24 hr by TRAP assay | Citation and Details Article DOI: 10.1007/s00044-011-9594-4 BindingDB Entry DOI: 10.7270/Q2X63QV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||