 Found 6 hits Enz. Inhib. hit(s) with Target = 'Thymidine kinase, cytosolic' and Ligand = 'BDBM1'
Found 6 hits Enz. Inhib. hit(s) with Target = 'Thymidine kinase, cytosolic' and Ligand = 'BDBM1' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thymidine kinase, cytosolic
(Homo sapiens (Human)) | BDBM1
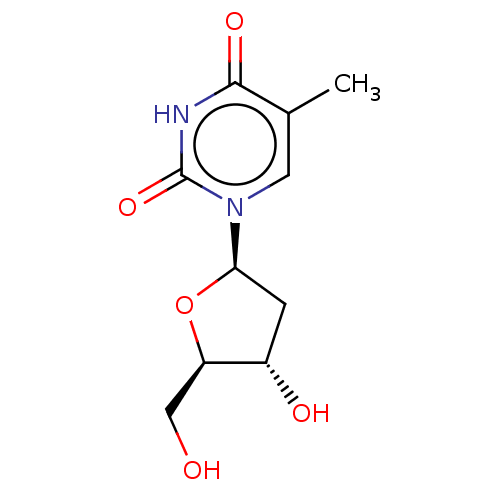
(dT | thymidine)Show SMILES Cc1cn([C@H]2C[C@H](O)[C@@H](CO)O2)c(=O)[nH]c1=O Show InChI InChI=1S/C6H10N6O/c1-12(2)11-10-6-4(5(7)13)8-3-9-6/h3,11H,1-2H3,(H2,7,13)/b10-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick
Curated by ChEMBL
| Assay Description
Binding affinity constant against HSV-1 thymidine kinase |
J Med Chem 46: 5045-54 (2003)
Article DOI: 10.1021/jm030241s
BindingDB Entry DOI: 10.7270/Q2BR8SXZ |
More data for this
Ligand-Target Pair | |
Thymidine kinase, cytosolic
(Homo sapiens (Human)) | BDBM1

(dT | thymidine)Show SMILES Cc1cn([C@H]2C[C@H](O)[C@@H](CO)O2)c(=O)[nH]c1=O Show InChI InChI=1S/C6H10N6O/c1-12(2)11-10-6-4(5(7)13)8-3-9-6/h3,11H,1-2H3,(H2,7,13)/b10-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Genetica Biochimica ed Evoluzionistica
Curated by ChEMBL
| Assay Description
Inhibition of HSV-1 thymidine kinase |
J Med Chem 35: 4214-20 (1992)
BindingDB Entry DOI: 10.7270/Q2J67HJP |
More data for this
Ligand-Target Pair | |
Thymidine kinase, cytosolic
(Homo sapiens (Human)) | BDBM1

(dT | thymidine)Show SMILES Cc1cn([C@H]2C[C@H](O)[C@@H](CO)O2)c(=O)[nH]c1=O Show InChI InChI=1S/C6H10N6O/c1-12(2)11-10-6-4(5(7)13)8-3-9-6/h3,11H,1-2H3,(H2,7,13)/b10-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC)
Curated by ChEMBL
| Assay Description
Inhibitory activity (50 uM) against HSV-1 Thymidine kinase in OST-TK-/HSV-1 TK+ cell line in combination with BVAraU |
J Med Chem 45: 4254-63 (2002)
BindingDB Entry DOI: 10.7270/Q2GQ6ZGB |
More data for this
Ligand-Target Pair | |
Thymidine kinase, cytosolic
(Homo sapiens (Human)) | BDBM1

(dT | thymidine)Show SMILES Cc1cn([C@H]2C[C@H](O)[C@@H](CO)O2)c(=O)[nH]c1=O Show InChI InChI=1S/C6H10N6O/c1-12(2)11-10-6-4(5(7)13)8-3-9-6/h3,11H,1-2H3,(H2,7,13)/b10-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC)
Curated by ChEMBL
| Assay Description
Inhibitory activity (50 uM) against HSV-1 Thymidine kinase in OST-TK-/HSV-1 TK+ cell line in combination with gancicclovir |
J Med Chem 45: 4254-63 (2002)
BindingDB Entry DOI: 10.7270/Q2GQ6ZGB |
More data for this
Ligand-Target Pair | |
Thymidine kinase, cytosolic
(Homo sapiens (Human)) | BDBM1

(dT | thymidine)Show SMILES Cc1cn([C@H]2C[C@H](O)[C@@H](CO)O2)c(=O)[nH]c1=O Show InChI InChI=1S/C6H10N6O/c1-12(2)11-10-6-4(5(7)13)8-3-9-6/h3,11H,1-2H3,(H2,7,13)/b10-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC)
Curated by ChEMBL
| Assay Description
Inhibitory concentration against HSV-1 TK (WT) catalyzed [3H]-GCV phosphorylation |
J Med Chem 45: 4254-63 (2002)
BindingDB Entry DOI: 10.7270/Q2GQ6ZGB |
More data for this
Ligand-Target Pair | |
Thymidine kinase, cytosolic
(Homo sapiens (Human)) | BDBM1

(dT | thymidine)Show SMILES Cc1cn([C@H]2C[C@H](O)[C@@H](CO)O2)c(=O)[nH]c1=O Show InChI InChI=1S/C6H10N6O/c1-12(2)11-10-6-4(5(7)13)8-3-9-6/h3,11H,1-2H3,(H2,7,13)/b10-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC)
Curated by ChEMBL
| Assay Description
Inhibitory concentration against HSV-1 TK (A167Y) catalyzed [3H]-GCV phosphorylation |
J Med Chem 45: 4254-63 (2002)
BindingDB Entry DOI: 10.7270/Q2GQ6ZGB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data