

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate synthase (Mus musculus) | BDBM50014480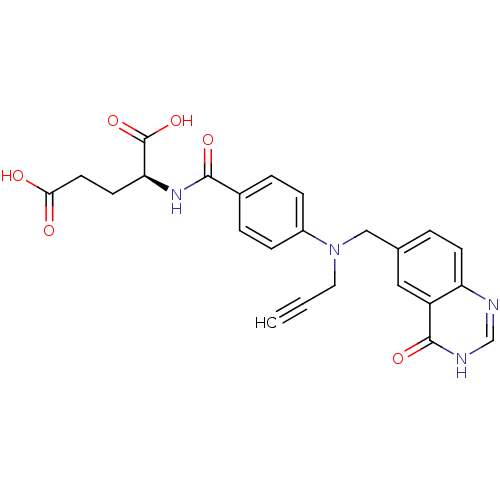 ((S)-2-(4-(((4-oxo-3,4-dihydroquinazolin-6-yl)methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside Curated by ChEMBL | Assay Description Concentration required for in vitro inhibition of thymidylate synthase | J Med Chem 33: 3060-7 (1990) BindingDB Entry DOI: 10.7270/Q2ZW1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50014480 ((S)-2-(4-(((4-oxo-3,4-dihydroquinazolin-6-yl)methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells overexpressing TS | J Med Chem 35: 859-66 (1992) BindingDB Entry DOI: 10.7270/Q2G44RG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50014480 ((S)-2-(4-(((4-oxo-3,4-dihydroquinazolin-6-yl)methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Mereside Curated by ChEMBL | Assay Description Concentration required for in vitro inhibition of thymidylate synthase | J Med Chem 33: 3060-7 (1990) BindingDB Entry DOI: 10.7270/Q2ZW1JWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50014480 ((S)-2-(4-(((4-oxo-3,4-dihydroquinazolin-6-yl)methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Concentration of the compound required to inhibit 50% activity of Thymidylate synthase was determined | J Med Chem 33: 3067-71 (1990) BindingDB Entry DOI: 10.7270/Q2V40T5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50014480 ((S)-2-(4-(((4-oxo-3,4-dihydroquinazolin-6-yl)methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Meerut Institute of Engineering and Technology Curated by ChEMBL | Assay Description Inhibition of thymidylate synthase | Eur J Med Chem 45: 1560-71 (2010) Article DOI: 10.1016/j.ejmech.2009.12.065 BindingDB Entry DOI: 10.7270/Q2D50P61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||