

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tumor susceptibility gene 101 protein (Homo sapiens (Human)) | BDBM50362889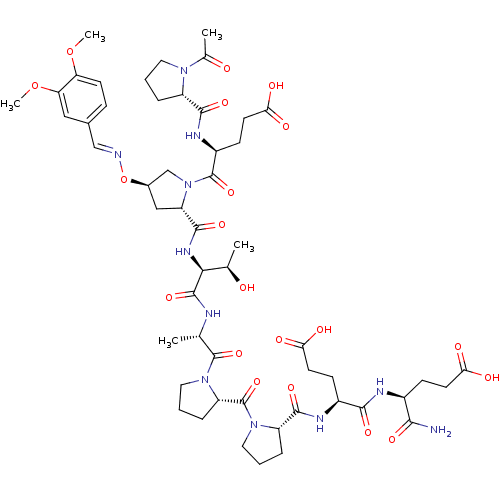 (CHEMBL1946129) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick Curated by ChEMBL | Assay Description Displacement of FITC-conjugated (S)-4-((S)-1-acetylpyrrolidine-2-carboxamido)-5-((2S,4R)-2-((2S,3R)-1-((S)-1-((S)-2-((S)-2-((S)-1-((S)-1-amino-4-carb... | ACS Med Chem Lett 2: 337-341 (2011) Article DOI: 10.1021/ml1002579 BindingDB Entry DOI: 10.7270/Q22B8ZF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor susceptibility gene 101 protein (Homo sapiens (Human)) | BDBM50362889 (CHEMBL1946129) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick Curated by ChEMBL | Assay Description Inhibition of human Tsg101 binding to GST-tagged P6 protein by surface plasmon resonance method | ACS Med Chem Lett 2: 337-341 (2011) Article DOI: 10.1021/ml1002579 BindingDB Entry DOI: 10.7270/Q22B8ZF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||