

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50383381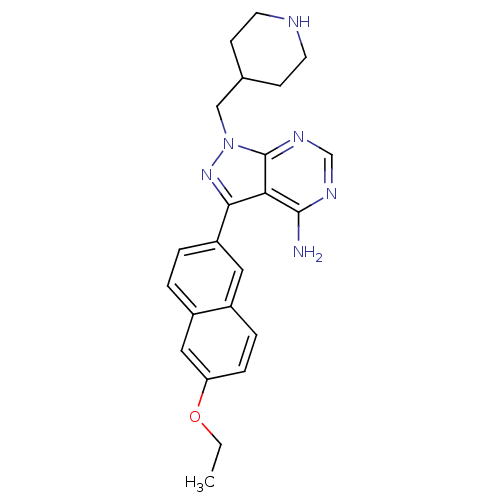 (CHEMBL2030554 | CHEMBL2070050 | US10544104, Compou...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human ABL by radiometric assay | J Med Chem 55: 2803-10 (2012) Article DOI: 10.1021/jm201725v BindingDB Entry DOI: 10.7270/Q2ST7QVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50383381 (CHEMBL2030554 | CHEMBL2070050 | US10544104, Compou...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human ABL using EAIYAAPFAKKK-OH as substrate by phosphorimaging method | J Med Chem 55: 2416-26 (2012) Article DOI: 10.1021/jm201713h BindingDB Entry DOI: 10.7270/Q2P2706V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50383381 (CHEMBL2030554 | CHEMBL2070050 | US10544104, Compou...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Through its Center for Commercialization US Patent | Assay Description Inhibition of human tyrosine kinases. | US Patent US9765037 (2017) BindingDB Entry DOI: 10.7270/Q2B56MVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50383381 (CHEMBL2030554 | CHEMBL2070050 | US10544104, Compou...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF WASHINGTON THROUGH ITS CENTER FOR CO US Patent | Assay Description Inhibition of TgCDPK1 and CpCDPK1 was determined using a luminescent kinase assay which measures ATP depletion in the presence of the Syntide 2 pepti... | US Patent US10544104 (2020) BindingDB Entry DOI: 10.7270/Q2D79DSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||